Abstract
Background:
Neurocognitive impairments of attention and executive functioning are trait abnormalities in schizophrenia, and these are considered to be endophenotypes. These deficits have been convincingly linked to prefrontal cortical functioning. In this study, we examined the cognitive performance in the domains of attention and executive functioning among first-degree relatives of Indian people with schizophrenia (high-risk [HR] patients) compared to healthy controls (HC).
Materials and Methods:
Siblings of patients with DSM-IV schizophrenia, HR patients (n = 17), were compared with HC (n = 30) (matched as a group for age, sex, years of education, and handedness) using the following neurocognitive tests for attention and executive function – digit span test (DST), trail making test, letter-number sequencing (LNS), and spatial span test.
Results:
HR patients had significantly deficient performance in attention and executive function tasks (DST-forward [P < 0.001], DST-backward [P < 0.001], spatial span-forward [P < 0.001], spatial span-backward [P < 0.001], and LNS [P < 0.001]).
Conclusions:
This study replicates the findings that neurocognitive deficits involving executive function task performance, attention, and working memory, which are considered as principal features in patients with schizophrenia, are also significantly present in the first-degree relatives of patients. Thus, these neurocognitive parameters can be considered as potential endophenotypes in schizophrenia.
Keywords: Attention, endophenotype, high-risk subjects, neurocognitive, schizophrenia
INTRODUCTION
Schizophrenia is a complex disorder with a multifactorial inheritance, and the presence of an affected first-degree relative is considered as a significant risk factor.[1] Neurocognitive deficits, which are consistently reported in schizophrenia patients, have been postulated as a potential endophenotype in schizophrenia.[2] Neurocognitive impairments have been demonstrated in all stages of schizophrenia and are intricately linked to the functional outcome of the illness.[3] Such cognitive deficits have also been demonstrated in remitted state in patients, and hence, these are considered to be “trait” factors.[4] These impairments are pronounced in the attention, processing speed, and executive functioning, in addition to verbal learning and memory.[5] These aberrations have also been convincingly linked to prefrontal cortical abnormalities associated with the illness.[6] Typically, the cognitive performance in patients with schizophrenia is[1,2] standard deviation less compared to matched healthy comparison controls.[7] Healthy first-degree relatives of patients or high-risk (HR) patients, such as siblings and offsprings of patients with schizophrenia, also demonstrate significant impairments in neurocognitive functioning,[8,9,10] indicating that such cognitive deficits could be potential endophenotypes of the illness.[11]
Prospective studies in HR patients have shown that verbal working memory, performance attention, and gross motor skills have predictive potential regarding conversion to psychosis.[12] Examining the cognitive endophenotypes in HR patients could help in evaluating the cognitive vulnerability markers without the effects of illness-related confounding factors. Previous studies including meta-analyses have demonstrated deficits in attention, working memory, and executive functioning in adult HR compared to healthy volunteers.[13,14,15] Studies from India have been sparse in this area. In a recent study from India, it was demonstrated that the siblings performed significantly poor as compared to the healthy controls (HCs) on Wisconsin card sorting test, continuous performance test, and spatial working memory test.[16] In this study, we sought to examine the neurocognitive performance in attention and executive functioning among siblings of patients with schizophrenia (HR) who attended a tertiary care psychiatric hospital in South India compared to matched HC.
MATERIALS AND METHODS
We recruited HR patients (n = 17) who are siblings of patients with DSM-IV schizophrenia who attended the clinical services of both inpatient and outpatient services of National Institute of Mental Health and Neurosciences, India. Age-, sex-, education-, and handedness-matched volunteering HCs (n = 30) were recruited through word of mouth. After complete description of the study to the participants, written informed consent was obtained. The Institute's Ethics Committee approved the study. All the participants were assessed using Mini International Neuropsychiatric Interview (MINI) Plus[17] to rule out the presence of axis I psychiatric diagnoses. At least one sibling with diagnosis of DSM-IV schizophrenia established by MINI and ascertained by a qualified psychiatrist was ensured for HR patients. None of these patients had clinical features suggestive of substance abuse/dependence. None had comorbid medical/neurological diagnosis. All the participants were right-handed as established using Edinburgh handedness inventory.[18] All the participants had formal education till at least 10th standard and had a score >25 on the Mini Mental Status Examination.[19]
Neurocognitive assessment was conducted for all the study participants in a single session (lasting for approximately 1 h), in a fixed order and in the same quiet room. The following neuropsychological tests were administered.
Tests of attention
-
Trail making test:[20] This examines for attention, sequencing, mental flexibility, and psychomotor speed (visual search and motor function) and consists of two parts:
- Part A – requires participant to make connection of circles containing numbers (digits) in ascending order, arranged randomly on paper
- Part B – requires participant to make connection of circles containing numbers (digits) and letters (alphabets) in alternating order, arranged randomly on paper.
Digit span test:[21] It consists of two subtests, namely digit-forward and digit-backward. Digit-forward contains eight items and digit-backward contains seven items, with each item containing two trials. In digit-forward, the participant is asked to read a sequence of digits and then asked to repeat the digits in the same order, while in digit-backward, in reverse order.
Tests of executive functions
Wechsler memory scale (WMS)-letter-number sequencing (LNS):[21] This measures working memory using auditory stimuli. The participant listens to a combination of numbers and letters and is asked to repeat them, saying the numbers first in an ascending order, and then the letters in the alphabetical order
WMS-spatial span:[21] This is a test for spatial working memory. The spatial span board has ten cubes and consists of two subtests – spatial span-forward and spatial span-backward. In spatial span-forward, the participant is asked to tap the same sequence as had been tapped by the examiner; while in the spatial span-backward, the participant has to tap the sequence in reverse order as had been tapped by the examiner.
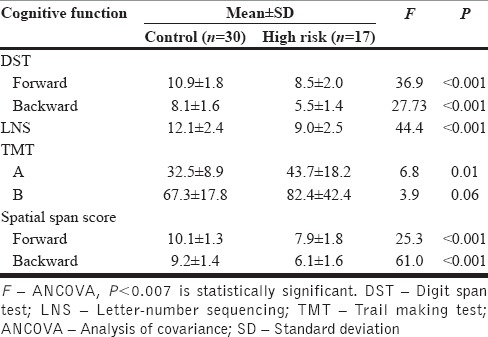
Analysis of covariance controlling for age, sex, and years of education was employed to examine the difference in neurocognitive task performance between HR and HC. Bonferroni corrected P < 0.007 was considered statistically significant, taking into account seven tests of comparison made between the groups [Table 1].
Table 1.
Comparison of attention and executive functions between high-risk patients and healthy controls
RESULTS
The HR patients were matched to HCs based on age (25.3 ± 4.6 vs. 24.9 ± 3.7, t = 0.3, P = 0.73), sex (male:female = 12:5 vs. 21:9, χ2 =0.02, P = 0.97), handedness (all subjects were right handed), and years of education (13.4 ± 2.5 vs. 13.1 ± 2.6, t = 0.3, P = 0.78). As shown in Table 1, HR patients had significantly deficient performance in attention and executive function tasks (digit span [forward] [P < 0.001], digit span [backward] [P < 0.001], spatial span [forward] [P < 0.001], spatial span [backward] [P < 0.001], and LNS [P < 0.001]).
DISCUSSION
In this study, HR patients demonstrated significant deficits in the neurocognitive measures of attention and executive functioning when compared with matched HCs.
Several previous studies have revealed that attention and executive functioning have been found to be among the most affected cognitive domains in schizophrenia.[14] Neurocognitive deficits involving executive function task performance, attention, and working memory could be considered as principal features in patients with schizophrenia since these aberrations are noticeable from the first episode of psychosis.[22] These domains are also found to be affected in first-degree relatives of patients with psychosis.[15] Examining the studies with HR patients (mean age between 15 and 29 years), Bora et al. in their recent meta-analysis reported that significant neurocognitive deficits are present in HR compared to HC in various domains.[23] In a recent study, Üçok et al. reported that HR patients performed poorly in attention, executive functions, and working memory than HC and the neurocognitive performance of HR was almost comparable to participants with the first episode psychosis.[24]
First-degree relatives of patients with schizophrenia or HR patients have been noted to have a significant predisposition to develop schizophrenia where some studies have noted the conversion rate as high as 40%.[25] Endophenotypes, also known as intermediate phenotypes, are measurable stable biological deficits or factors which could indicate inherited vulnerability to the disease in question. Hence, they co-segregate within the families of patients,[26] and as replicated in this study, neurocognitive impairments involving attention and executive functions can be considered to be endophenotypes.
Schizophrenia is probably not understood as dysfunction due to single brain area; rather, it is considered to be a disorder of brain networks.[27] Many brain areas such as prefrontal cortex, hippocampus, and parietal lobe structures are consistently reported to be associated with cognitive function deficits, especially the attention and executive function impairments.[28,29,30] It has been shown that the vulnerability to develop schizophrenia might reveal regional gray matter density alterations in functionally relevant brain circuits, especially involving the prefrontal regions.[31] Neuro-hemodynamic changes involving frontal brain regions which form a part of the attentional network has been found to be an important endophenotype marker of schizophrenia.[32] Indeed, many of the frontal lobe-mediated neurocognitive functions have been found to have significant heritability estimates.[33] Albeit in a relatively smaller number of HR patients, our study further adds evidence supporting that attention and executive functioning are cognitive endophenotypes of schizophrenia.
Financial support and sponsorship
This work is supported by the CEIB Programme Support Grant to G.V. (BT/PR5322/COE/34/8/2012).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work is supported by the CEIB Programme Support Grant to G.V. (BT/PR5322/COE/34/8/2012).
REFERENCES
- 1.Waddington JL, Corvin AP, Donohoe G, O’Tuathaigh CM, Mitchell KJ, Gill M. Functional genomics and schizophrenia: Endophenotypes and mutant models. Psychiatr Clin North Am. 2007;30:365–99. doi: 10.1016/j.psc.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Allen AJ, Griss ME, Folley BS, Hawkins KA, Pearlson GD. Endophenotypes in schizophrenia: A selective review. Schizophr Res. 2009;109:24–37. doi: 10.1016/j.schres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lepage M, Bodnar M, Bowie CR. Neurocognition: Clinical and functional outcomes in schizophrenia. Can J Psychiatry. 2014;59:5–12. doi: 10.1177/070674371405900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rund BR. A review of longitudinal studies of cognitive functions in schizophrenia patients. Schizophr Bull. 1998;24:425–35. doi: 10.1093/oxfordjournals.schbul.a033337. [DOI] [PubMed] [Google Scholar]
- 5.Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;213:11–37. doi: 10.1007/978-3-642-25758-2_2. [DOI] [PubMed] [Google Scholar]
- 6.Conklin HM, Curtis CE, Calkins ME, Iacono WG. Working memory functioning in schizophrenia patients and their first-degree relatives: Cognitive functioning shedding light on etiology. Neuropsychologia. 2005;43:930–42. doi: 10.1016/j.neuropsychologia.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57:688–91. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: A quantitative and qualitative review. Cogn Neuropsychiatry. 2013;18:44–82. doi: 10.1080/13546805.2012.676309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson H, Cullen AE, Reichenberg A, Hodgins S, Campbell DD, Morris RG, et al. Cognitive impairment among children at-risk for schizophrenia. J Psychiatr Res. 2014;50:92–9. doi: 10.1016/j.jpsychires.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Keshavan MS, Kulkarni S, Bhojraj T, Francis A, Diwadkar V, Montrose DM, et al. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Neurosci. 2010;3:62. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: An overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlenmeyer-Kimling L. Neurobehavioral deficits in offspring of schizophrenic parents: Liability indicators and predictors of illness. Am J Med Genet. 2000;97:65–71. doi: 10.1002/(sici)1096-8628(200021)97:1<65::aid-ajmg9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–58. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 14.Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: A meta-analysis. Schizophr Res. 2004;71:285–95. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–94. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg R, Trivedi JK, Dalal PK, Nischal A, Sinha PK, Varma S. Assessment of cognition in non-affected full biological siblings of patients with schizophrenia. Indian J Psychiatry. 2013;55:331–7. doi: 10.4103/0019-5545.120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 18.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale. 3rd ed. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 22.Ma X, Wang Q, Sham PC, Liu X, Rabe-Hesketh S, Sun X, et al. Neurocognitive deficits in first-episode schizophrenic patients and their first-degree relatives. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:407–16. doi: 10.1002/ajmg.b.30330. [DOI] [PubMed] [Google Scholar]
- 23.Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C. Cognitive deficits in youth with familial and clinical high risk to psychosis: A systematic review and meta-analysis. Acta Psychiatr Scand. 2014;130:1–15. doi: 10.1111/acps.12261. [DOI] [PubMed] [Google Scholar]
- 24.Üçok A, Direk N, Koyuncu A, Keskin-Ergen Y, Yüksel Ç, Güler J, et al. Cognitive deficits in clinical and familial high risk groups for psychosis are common as in first episode schizophrenia. Schizophr Res. 2013;151:265–9. doi: 10.1016/j.schres.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: Psychopathology and clinical features. Schizophr Res. 2004;67:131–42. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 26.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 27.Mesulam MM. Schizophrenia and the brain. N Engl J Med. 1990;322:842–5. doi: 10.1056/NEJM199003223221209. [DOI] [PubMed] [Google Scholar]
- 28.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–49. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro ML, Eichenbaum H. Hippocampus as a memory map: Synaptic plasticity and memory encoding by hippocampal neurons. Hippocampus. 1999;9:365–84. doi: 10.1002/(SICI)1098-1063(1999)9:4<365::AID-HIPO4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 30.Hart SJ, Bizzell J, McMahon MA, Gu H, Perkins DO, Belger A. Altered fronto-limbic activity in children and adolescents with familial high risk for schizophrenia. Psychiatry Res. 2013;212:19–27. doi: 10.1016/j.pscychresns.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habets P, Krabbendam L, Hofman P, Suckling J, Oderwald F, Bullmore E, et al. Cognitive performance and grey matter density in psychosis: Functional relevance of a structural endophenotype. Neuropsychobiology. 2008;58:128–37. doi: 10.1159/000182889. [DOI] [PubMed] [Google Scholar]
- 32.Filbey FM, Russell T, Morris RG, Murray RM, McDonald C. Functional magnetic resonance imaging (fMRI) of attention processes in presumed obligate carriers of schizophrenia: Preliminary findings. Ann Gen Psychiatry. 2008;7:18. doi: 10.1186/1744-859X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–9. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]


