Abstract
Recent years have witnessed critical contributions to our understanding of the determinants and long-term implications of lung function development. In this article, we review studies that have contributed to advances in understanding lung function development and its critical importance for lung health into adult life. In particular, we have focused on early life determinants that include genetic factors, perinatal events, environmental exposures, lifestyle, infancy lower respiratory tract infections, and persistent asthma phenotypes. Longitudinal studies have conclusively demonstrated that lung function deficits that are established by school age may track into adult life and increase the risk of adult lung obstructive diseases, such as chronic obstructive pulmonary disease. Furthermore, these contributions have provided initial evidence in support of a direct influence by early life events on an accelerated decline of lung function and an increased susceptibility to its environmental determinants well into adult life. As such, we argue that future health-care programs based on precision medicine approaches that integrate deep phenotyping with tailored medication and advice to patients should also foster optimal lung function growth to be fully effective.
Keywords: Asthma, children, COPD, FEV1, genetics, lung function, tobacco smoke, trajectories
Introduction
Lung development starts in utero and may continue throughout childhood 1, 2. Evidence is now emerging that several chronic adult diseases—including chronic obstructive pulmonary disease (COPD), which is estimated by the World Health Organization (WHO) to become the third leading cause of death worldwide by 2030 3—may have part of their origins early in life 4– 9. These observations fit well with the Developmental Origins of Health and Disease (DOHaD) concept that describes how early life exposures may have a long-term impact on diseases in adulthood 5, 6, 10– 12. Within this framework, we review studies that have contributed to recent advances in understanding lung function development and its critical importance for lung health into adult life. The review covers key determinants, from genetics to early life events and environmental exposures, and focuses primarily on lung function trajectories from childhood to adulthood.
Factors affecting lung function growth
Genetic factors influencing lung function
The genetic determinants for lung function have been evaluated in numerous candidate gene studies over the years and in the last 5 to 10 years successfully in large genome-wide association studies (GWASs). In the most recent GWAS on almost 50,000 subjects from the UK Biobank followed by replication in 95,000, it was concluded that the number of independent genetic associations with any lung function parameter—forced expiratory volume in one second (FEV 1), forced vital capacity (FVC), or the FEV 1/FVC ratio—is now 97, representing loci across the whole genome 13. The total heritability explained by these 97 signals was estimated to 9.6% for FEV 1, 6.4% for FVC, and 5.2% for FEV 1/FVC. Importantly, most of the identified single-nucleotide polymorphisms (SNPs) seem to influence lung function in both children and adults, a pattern that has been observed in several studies 14– 18. Many of the identified SNPs have also been associated with COPD in previous studies 19. Attempts have been made to identify gene variants predisposing to different lung function trajectories from childhood to adulthood, and in the US CAMP study a SNP on chromosome 8 (rs4445257) between CSMD3 and TRPS1 was found to be significantly associated with a normal-growth, early-decline pattern 20. However, in adults, genetic variants known to be strongly associated with cross-sectional lung function show little or no association with the rate of lung function decline over time 21.
Recent understanding that the genetic determinants of lung function operate across the life cycle lends support to the hypothesis that lung function trajectories from childhood to adulthood are at least partly defined at birth and early life. Intriguingly, pathway analyses show enrichment in lung function genes for developmental processes, and functional genetic and proteomic analyses of fetal lung samples also show that several of these genes (for example, TMEM163, FAM13A, HHIP, CDC123, PTCH1, and RAGE) affect lung development already at the embryonic stage 22, 23. As such, it is possible that variants associated with lung function and respiratory disease in adulthood may actually influence risk through mechanisms that are at least partly related to lung development.
The relation of preterm birth to lung function
Undisputable examples of early life effects are chronic lung disease (for example, bronchopulmonary dysplasia) and lung function impairment in individuals born very prematurely (fewer than 32 gestational weeks) 24– 27. Recent studies also show that late to moderate preterm birth (32 to 36 gestational weeks) is associated with significant lung function deficits at least up to adolescence, particularly for airflow limitation indices measured as FEV 1 and FEV 1/FVC 11, 12. Lung function catch-up (that is, recovery of deficits observed during childhood) has been reported in some 28, 29 but not all 11 studies. The long-term clinical relevance of small to moderate lung function deficits in childhood related to preterm birth and perinatal events is not known. However, concern has been raised as to whether individuals born preterm, especially those born extremely preterm, may be at risk of developing COPD-like phenotypes later in life 30. In addition, it is still unclear whether preterm birth is associated with a more rapid age-related decline in lung function in adulthood.
Relevance of environmental exposures
Air pollutants may induce airway inflammation, increased airway responsiveness, and lung damage and this is partly due to generation of free radicals and oxidative stress. Exposure to traffic-related air pollution has been negatively associated with lung growth and lung function (primarily FEV 1) in children and young adults in several studies, leading to increased risk of clinically important deficits 31– 34. In studies from the Swedish BAMSE (Barn/children Allergy Milieu Stockholm Epidemiology) cohort, conducted in the Stockholm area with air pollution exposure levels well below the current WHO guidelines, exposure during the first year of life seemed to have the largest impact on later lung function 31, 32, 35. Early life exposure is also associated with increased risk of asthma throughout childhood 36, and interaction with genetic factors related to COPD has recently been reported 37. These studies indicate a vulnerable time window early in life, and this is consistent with the DOHaD hypothesis. However, it is still unclear whether early life exposure has long-term effects into adulthood. Reports from the Children’s Health Study in California show convincing data on the negative impact on lung function indices from air pollution exposures later during childhood and adolescence 38, 39. Notably, improvements in air quality have been shown to have positive effects on lung function growth between 11 and 15 years, indicating that later exposures are very likely to be of importance 40.
One of the most well-studied risk factors for respiratory disease is tobacco smoke exposure. Several studies have reported maternal smoking during pregnancy as a major risk factor for impaired lung development 41– 43. The epidemiological associations have been supported by experimental studies showing structural lung defects, hyperplasia of neuro-endocrine cells, and decreased lung growth in offspring exposed to tobacco smoke in utero 44, 45 as well as human data reporting consistently altered epigenetic profiles in children of mothers who smoked during pregnancy 46. Secondhand tobacco exposure later during childhood has also been associated with persistence of respiratory symptoms into adult life 47, 48. Indeed, studies reporting interactive effects between parental and active smoking in affecting FEV 1 decline and COPD risk are among the best illustrative examples of how risk factors from early and adult life may have detrimental joint health effects. In cross-sectional studies of active smokers, having a mother who also smoked was linked to airflow limitation 49 and early-onset 50 and severe 51 COPD and these observations have been recently expanded to decline of lung function in longitudinal studies, as described in the following sections. Despite these well-known negative health effects and anti-smoking campaigns worldwide, smoking during pregnancy and elsewhere remains a major public health challenge.
Dietary factors and physical activity
Our constantly changing lifestyle has a broad impact on health and well-being. Diet and physical activity are modifiable factors that may influence lung development and respiratory disease across the life span. Systematic reviews show evidence of a beneficial effect of fresh fruits, and antioxidant vitamins on recurrent wheeze and asthma, but most evidence stems from cross-sectional studies and there is a need for more well-designed randomized controlled trials (RCTs) 52. In particular, vitamin D has been implicated to have a key role in lung development 53, and maternal deficiency has been reported to be associated with impaired lung development in school-aged offspring 54, 55. Although recent RCTs have shown that fish oil-derived fatty acid, vitamin C, or vitamin D supplementation during pregnancy may have beneficial effects on offspring respiratory health 56– 59, there are currently no consensus guidelines or recommendations of specific diets or supplements for lung function improvement in children. Results from ongoing clinical studies in this area are much anticipated.
Physical activity and fitness have been associated with childhood lung function in some 60, 61, but not all 62 studies. However, recent longitudinal data show that achieving increased fitness from young adulthood to middle age is associated with less decline in lung health over time 63. These results are encouraging for patients with a lung disease.
Role of lower respiratory tract infections
From a clinical point of view, it is well known that infants with severe bronchiolitis triggered by, for example, respiratory syncytial virus (RSV) or rhinovirus (RV) are at risk of later asthma or lung function impairment, or both. Longitudinal cohorts show that children with virus-induced wheezing symptoms during the first years of life may outgrow their symptoms later in childhood but as a group do not completely overcome their lung function impairment 64– 70. If a child develops recurrent wheeze or asthma following lower respiratory tract infection (LRTI) in infancy, he or she may be at increased risk for a further deterioration of lung function (the two-hit hypothesis 65, 71, 72) or develop an increased susceptibility to later noxious environmental exposures as discussed below. Whether these early and later lung function insults are related to independent risk factors and pathways or instead share a genetically determined susceptibility remains to be elucidated.
The underlying mechanism for the association between respiratory tract infection in early life and later respiratory morbidity is not clear. Proposed mechanisms include modulation of the immune response, direct airway damage, pre-existing deficits in lung function, and genetic susceptibility—highlighting different aspects of cause and effect 73– 77. Some studies suggest that specifically an early RV wheezing attack or bronchiolitis event is a marker of risk for later asthma 78, whereas others suggest that the number, not the particular viral species, of LRTI episodes in the first years of life is of primary importance for later asthma development 79. As a possible mechanism linking RV to asthma, the gene encoding for the RV receptor (C) is the asthma susceptibility gene CDHR3 on chromosome 7q22. CDHR3 was first identified in a GWAS on severe asthma in children 80, and only later did experimental work lead to the identification of CDHR3 as the virus receptor 81. In addition, variants at the 17q21 asthma locus ( ORMDL3) have been specifically associated with asthma in children who had had RV wheezing illnesses early in life, connecting genetic susceptibility, RV infection, and asthma development 82.
Lung function trajectories from childhood into adult life
Long-term effects of lung function development into adult life
A large growing body of evidence indicates that lung function development in utero, infancy, and childhood may have long-lasting effects on respiratory health throughout the life span. Most studies have addressed trajectories of lung function from childhood into adult life by focusing on FEV 1 and the FEV 1/FVC ratio as indices of airflow limitation. Consequently, airway obstruction—which is defined by an abnormally low FEV 1/FVC ratio and represents the hallmark of COPD 4—has been by far the most extensively studied spirometric pattern and will be the main focus of this section, although we argue that studies addressing the early origins of spirometric restriction 83– 86 are also needed because of its remarkable prevalence, morbidity, and mortality burden.
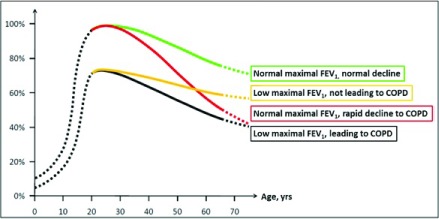
The possible contribution of childhood factors on the natural history of obstructive lung diseases across the life span has been the topic of much debate for decades 87– 90. In recent years, the importance of a full growth to maximal lung function in childhood has been reinforced by conclusive evidence that COPD can develop in mid to late adult life through at least two main trajectories: by the classic trajectory of an accelerated FEV 1 decline in adulthood following a normal lung function development in childhood (“Rapid FEV 1 decline” trajectory, red line in Figure 1, modified from reference 7) or alternatively by a low maximal lung function attained by the beginning of adult life without necessarily an accelerated FEV 1 decline thereafter (“Low maximal FEV 1” trajectory, black line in Figure 1). Strikingly, in a recent study 7 that included three large prospective cohorts, about 50% of participants with COPD exhibited features compatible with the latter trajectory, suggesting that lung function development in childhood may play a substantially more relevant role in COPD susceptibility than traditionally thought.
Figure 1. Lung function trajectories to chronic obstructive pulmonary disease (COPD).
The figure represents four lung function trajectories identified in the study by Lange et al. 7 based on levels of forced expiratory volume in one second (FEV 1) before the age of 40 years (below or above 80% of predicted value) and the presence or absence of Global initiative for chronic Obstructive Lung Disease (GOLD) grade of at least 2 COPD at the end of follow-up. The y-axis represents the percentage of expected maximally attained FEV 1. Modified from 7.
Early origins of the low maximal lung function trajectory
Among the characteristics associated with a persistently low lung function trajectory are the presence of recurrent wheezing, asthma, and asthma-related phenotypes in childhood. As several longitudinal birth cohorts have now entered their adult years, their findings have been consistent in showing substantial tracking of asthma-related lung function deficits from childhood into adulthood but no evidence of accelerated decline thereafter 91, 92. Notably, studies that have measured indices of airway resistance and airflow at earlier ages 71, 72 have demonstrated that, although deficits of lung function can be detected as early as one month after birth, most children who have persistent wheezing and asthma by early school age experience a progressive deterioration of their lung function deficits as they transition from infancy to school age. These findings suggest that in utero life, infancy, and early childhood may be critical windows of opportunity for early prevention of long-term sequelae of childhood asthma on lung health.
In this context, several recent studies have provided strong evidence that the lung function deficits associated with severe childhood asthma may lead in a subgroup of patients to the development of COPD. In the Melbourne study 93 and Scottish WHEASE (What Happens Eventually to Asthmatic children: Sociologically and Epidemiologically) cohort 94, children with asthma—particularly if severe—had a striking increase in the risk of having a post-bronchodilator FEV 1/FVC ratio of less than 70% by the age of 50 to 65 years. Consistent with these epidemiological data, up to 11% of children with persistent mild to moderate asthma in the clinical CAMP study were found to develop COPD according to spirometric criteria by age 30 years 8. In that study 8, participants were classified into four groups on the basis of visual inspection of their lung function trajectories from childhood into young adult life: normal growth, normal growth and early decline, reduced growth, and reduced growth plus early decline. In line with findings from the studies described above, nearly 85% of the cases who went on to develop COPD by young adulthood occurred among participants who experienced a reduced lung function growth in childhood and adolescence. Interestingly, despite the relatively short follow-up in adulthood, an early start of lung function decline appeared to also carry an increase in COPD risk. Future studies should address to what extent a short plateau phase or early decline of lung function may also contribute to COPD development among patients with asthma.
It should be noted that the tracking of lung function from childhood into adult life is not observed only among individuals with asthma but has been repeatedly shown in samples from the general population. Tasmanian children who were in the lowest quartile of FEV 1/FVC at age 7 years had a six- to 16-fold increase in their odds for COPD in the absence or presence of concomitant asthma by age 45 9. In the population-based Tucson Children’s Respiratory Study, participants in the lowest quartile of airway function in early infancy had significantly lower values for FEV 1 and FEV 1/FVC up to age 22 as compared with participants in the upper three quartiles 95, and a distinct group of individuals with persistently low lung function between the ages of 11 and 32 years (a large proportion of whom did not have asthma) could be identified by using latent class analysis 10. Interestingly, participants in this impaired lung function trajectory, as compared with participants in the normal lung function trajectory, were nearly twice as likely to have had RSV lower respiratory tract infections in the first three years of life. Thus, we argue that multiple host factors, exposures, and events that have a direct impact on lung function at any developmental stage may in principle contribute to put a child in a trajectory of persistently low lung function into adult life, with a very broad range of effect magnitude. What makes some children robust to the effects of these risk factors and why some children may develop only initial lung function deficits in response to these exposures that are transient and eventually overcome them as they enter adult life remain largely unknown and, as discussed in the concluding section, a question with critical implications for prevention.
Early origins of rapid lung function decline
Evidence is beginning to emerge that early life factors may also predispose to an accelerated decline of lung function in adult life (red line in Figure 1). Among adult participants in the European Community Respiratory Health Survey, recalling the presence of “disadvantage” factors in childhood (that is, maternal asthma, paternal asthma, childhood asthma, maternal smoking, or childhood respiratory infections or a combination of these) was associated not only with the presence of airflow limitation but also with an accelerated decline of FEV 1 96. Similarly, in the adult CARDIA (Coronary Artery Risk Development in Young Adults) Study, a low childhood socioeconomic status (as assessed by self-reported parental education) was associated with a steeper decline of both FEV 1 and FVC in adult life, although the specific factor(s) involved in explaining this association could not be identified 97.
Despite this evidence, data in support of direct effects of early life factors on accelerated decline of lung function in adult life are still sparse and consequently the magnitude and consistency of these associations unclear. This may be due to the inherent methodological difficulties of assessing these effects within a longitudinal study design or to the possibility that these effects are synergistic with other exposures in adult life. Although both scenarios are likely to be correct, the latter is supported by the recent and growing body of evidence from epidemiological and experimental studies suggesting that early life factors may increase susceptibility to the effects of adult life exposures in affecting lung disease. The presence of RSV LRTIs in the first 3 years of life and other childhood factors have been shown to interact with active smoking and occupational hazards in adulthood to affect respiratory symptoms and asthma risk 98, 99. Most interestingly, early exposure to maternal and parental smoking has been shown to enhance susceptibility to smoking-related accelerated decline of lung function in adulthood 100, 101. In the Tucson cohort, participants were classified on the basis of exposure to parental smoking (assessed at birth) and active smoking in adult life 101. Between the ages of 11 and 26 years, participants with exposure to both parental and active smoking had the steepest decline in sex-, age-, and height-adjusted residuals of FEV 1/FVC, FEV 1, forced expiratory flow at 25% to 75% of FVC (FEF 25–75), and FEF 25–75/FVC. In contrast, no significant deficits were seen at this young age among participants who were exposed to only parental or only active smoking, indicating that early life (or in utero) exposure to environmental tobacco smoke increases susceptibility to the deleterious effects that active adult smoking will have on lung health. The exact mechanisms through which exposures to tobacco smoke in early and adult life interact with each other in affecting susceptibility remain largely unknown, as does the extent to which these synergist effects with adult life exposures may apply to other early life factors. Epidemiological and clinical studies aimed to dissect systematically interactive effects between early and adult life events on lung health outcomes are warranted.
Conclusions
Recent years have witnessed critical contributions to our understanding of the determinants and long-term implications of lung function development. In addition to further delineating the role of genetics, perinatal events, childhood environmental exposures, lifestyle, infancy LRTIs, and persistent asthma phenotypes, these contributions have conclusively demonstrated that lung function deficits that are established by school age may track into adult life and increase the risk of adult lung obstructive diseases, including COPD. Furthermore, these contributions have provided initial evidence in support of a direct influence by early life events on an accelerated decline of lung function and an increased susceptibility to its environmental determinants (for example, tobacco smoke) well into adult life. Thus, this evidence indicates early life as a critical time that may contribute to set the pace of lung aging processes that will take place several decades later.
Although this conclusion highlights the critical importance of developmental age, it should not undermine in any way the importance of behavioral and environmental risk factors for obstructive lung diseases that take place in adult life. Indeed, avoidance of such risk factors in both childhood and adulthood needs to play a critical role in primary to tertiary prevention. We argue that tailoring risk profiles and intervention strategies based on information from both early and adult life factors may provide a significant improvement in the way we prevent and treat lung disease.
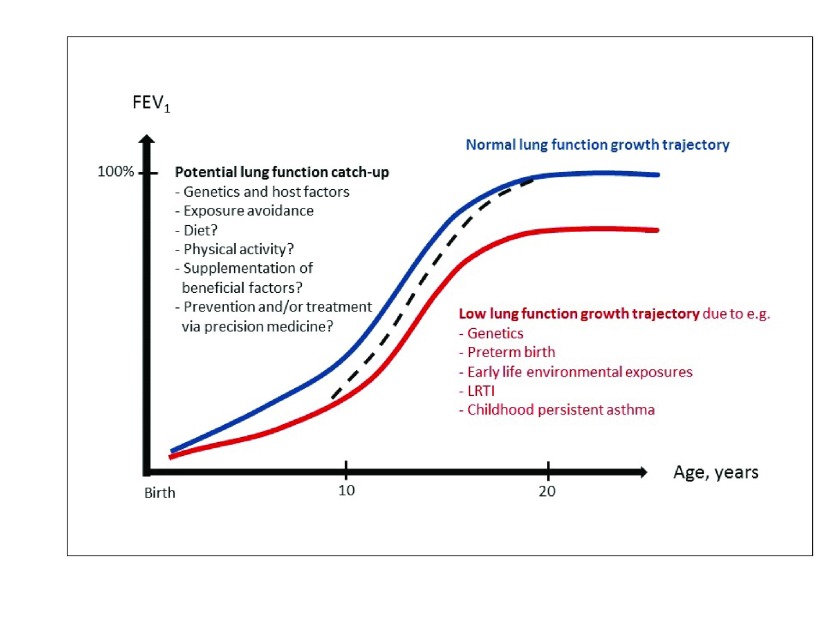
As a concluding note, we point out that although great effort has been directed to characterize trajectories of lung function deficits and to identify risk factors associated with lung function impairment, less is known about factors influencing optimal lung function growth or recovery from early deficits or after early insults such as preterm birth or a severe LRTI ( Figure 2). Indeed, whether (and to what extent) lung function catch-up occurs in groups of children with early deficits has been insufficiently studied to date. Identifying effective strategies—apart from avoiding obvious risk factors like tobacco smoke, air pollution exposure, and recurrent respiratory infections—to enhance this early catch-up would have critical implications. The roles of dietary components and supplementations 52, 53 and those of physical activity and fitness 60– 62 are among those being investigated. Molecules that may play a direct protective role in the lung—such as the club cell secretory protein (CC16)—are also being evaluated in epidemiological 102, 103 and clinical 104, 105 studies and may hold promise as future therapeutic strategies.
Figure 2. Lung function trajectories from childhood to adult life.
The blue line represents a normal lung function growth, the red line represents a low lung function trajectory and associated risk factors, and the black dotted line represents lung function catch-up from childhood to adulthood. Potential beneficial factors for lung function catch-up are listed in black text. FEV 1, forced expiratory volume in one second; LRTI, lower respiratory tract infection.
In this context, the major advances from recent years in understanding the genetic and molecular components of lung function not only will pave the way for new asthma and COPD drugs but also provide critical knowledge to help doctors and caregivers to optimize their patients’ lung health. Future health-care programs based on precision medicine approaches that integrate deep phenotyping with tailored medication and advice to patients should also foster optimal lung function growth to be fully effective.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
David Mannino, University of Kentucky School of Medicine, Lexington, KY, USA
Scott Weiss, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA
Funding Statement
EM is supported by research grants from the Swedish Heart-Lung Foundation, the Swedish Research Council, the Stockholm County Council (ALF), and the Strategic Research Programme (SFO) in Epidemiology at Karolinska Institutet. SG is supported by research grants from the National Institutes of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Yammine S, Schmidt A, Sutter O, et al. : Functional evidence for continued alveolarisation in former preterms at school age? Eur Respir J. 2016;47(1):147–55. 10.1183/13993003.00478-2015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Butler JP, Loring SH, Patz S, et al. : Evidence for adult lung growth in humans. N Engl J Med. 2012;367(3):244–7. 10.1056/NEJMoa1203983 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. World Health Organization;2007. Reference Source [Google Scholar]
- 4. Vogelmeier CF, Criner GJ, Martinez FJ, et al. : Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–82. 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 5. Hanson M, Gluckman P: Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr. 2011;94(6 Suppl):1754S–1758S. 10.3945/ajcn.110.001206 [DOI] [PubMed] [Google Scholar]
- 6. Martinez FD: Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2016;375(9):871–8. 10.1056/NEJMra1603287 [DOI] [PubMed] [Google Scholar]
- 7. Lange P, Celli B, Agusti A, et al. : Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373(2):111–22. 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 8. McGeachie MJ, Yates KP, Zhou X, et al. : Patterns of Growth and Decline in Lung Function in Persistent Childhood Asthma. N Engl J Med. 2016;374(19):1842–52. 10.1056/NEJMoa1513737 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Bui DS, Burgess JA, Lowe AJ, et al. : Childhood Lung Function Predicts Adult COPD and Asthma-COPD Overlap Syndrome (ACOS). Am J Respir Crit Care Med. 2017. 10.1164/rccm.201606-1272OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Berry CE, Billheimer D, Jenkins IC, et al. : A Distinct Low Lung Function Trajectory from Childhood to the Fourth Decade of Life. Am J Respir Crit Care Med. 2016;194(5):607–12. 10.1164/rccm.201604-0753OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thunqvist P, Gustafsson PM, Schultz ES, et al. : Lung Function at 8 and 16 Years After Moderate-to-Late Preterm Birth: A Prospective Cohort Study. Pediatrics. 2016;137(4): pii: e20152056. 10.1542/peds.2015-2056 [DOI] [PubMed] [Google Scholar]
- 12. den Dekker HT, Sonnenschein-van der Voort AM, de Jongste JC, et al. : Early growth characteristics and the risk of reduced lung function and asthma: A meta-analysis of 25,000 children. J Allergy Clin Immunol. 2016;137(4):1026–35. 10.1016/j.jaci.2015.08.050 [DOI] [PubMed] [Google Scholar]
- 13. Wain LV, Shrine N, Artigas MS, et al. : Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat Genet. 2017;49(3):416–25. 10.1038/ng.3787 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Hunninghake GM, Cho MH, Tesfaigzi Y, et al. : MMP12, lung function, and COPD in high-risk populations. N Engl J Med. 2009;361(27):2599–608. 10.1056/NEJMoa0904006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Loth DW, Soler Artigas M, Gharib SA, et al. : Genome-wide association analysis identifies six new loci associated with forced vital capacity. Nat Genet. 2014;46(7):669–77. 10.1038/ng.3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soler Artigas M, Wain LV, Miller S, et al. : Sixteen new lung function signals identified through 1000 Genomes Project reference panel imputation. Nat Commun. 2015;6: 8658. 10.1038/ncomms9658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wain LV, Shrine N, Miller S, et al. : Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med. 2015;3(10):769–81. 10.1016/S2213-2600(15)00283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Panasevich S, Melén E, Hallberg J, et al. : Investigation of novel genes for lung function in children and their interaction with tobacco smoke exposure: a preliminary report. Acta Paediatr. 2013;102(5):498–503. 10.1111/apa.12204 [DOI] [PubMed] [Google Scholar]
- 19. Cho MH, McDonald ML, Zhou X, et al. : Risk loci for chronic obstructive pulmonary disease: a genome-wide association study and meta-analysis. Lancet Respir Med. 2014;2(3):214–25. 10.1016/S2213-2600(14)70002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. McGeachie MJ, Yates KP, Zhou X, et al. : Genetics and Genomics of Longitudinal Lung Function Patterns in Individuals with Asthma. Am J Respir Crit Care Med. 2016;194(12):1465–74. 10.1164/rccm.201602-0250OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. John C, Soler Artigas M, Hui J, et al. : Genetic variants affecting cross-sectional lung function in adults show little or no effect on longitudinal lung function decline. Thorax. 2017;72(5):400–8. 10.1136/thoraxjnl-2016-208448 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Miller S, Henry AP, Hodge E, et al. : The Ser82 RAGE Variant Affects Lung Function and Serum RAGE in Smokers and sRAGE Production In Vitro. PLoS One. 2016;11(10):e0164041. 10.1371/journal.pone.0164041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller S, Melén E, Merid SK, et al. : Genes associated with polymorphic variants predicting lung function are differentially expressed during human lung development. Respir Res. 2016;17(1):95. 10.1186/s12931-016-0410-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Broström EB, Thunqvist P, Adenfelt G, et al. : Obstructive lung disease in children with mild to severe BPD. Respir Med. 2010;104(3):362–70. 10.1016/j.rmed.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 25. Doyle LW, Faber B, Callanan C, et al. : Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118(1):108–13. 10.1542/peds.2005-2522 [DOI] [PubMed] [Google Scholar]
- 26. Filippone M, Bonetto G, Cherubin E, et al. : Childhood course of lung function in survivors of bronchopulmonary dysplasia. JAMA. 2009;302(13):1418–20. 10.1001/jama.2009.1419 [DOI] [PubMed] [Google Scholar]
- 27. Vollsæter M, Røksund OD, Eide GE, et al. : Lung function after preterm birth: development from mid-childhood to adulthood. Thorax. 2013;68(8):767–76. 10.1136/thoraxjnl-2012-202980 [DOI] [PubMed] [Google Scholar]
- 28. Kotecha SJ, Dunstan FD, Kotecha S: Long term respiratory outcomes of late preterm-born infants. Semin Fetal Neonatal Med. 2012;17(2):77–81. 10.1016/j.siny.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 29. Narang I, Bush A: Early origins of chronic obstructive pulmonary disease. Semin Fetal Neonatal Med. 2012;17(2):112–8. 10.1016/j.siny.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 30. Broström EB, Akre O, Katz-Salamon M, et al. : Obstructive pulmonary disease in old age among individuals born preterm. Eur J Epidemiol. 2013;28(1):79–85. 10.1007/s10654-013-9761-7 [DOI] [PubMed] [Google Scholar]
- 31. Schultz ES, Gruzieva O, Bellander T, et al. : Traffic-related air pollution and lung function in children at 8 years of age: a birth cohort study. Am J Respir Crit Care Med. 2012;186(12):1286–91. 10.1164/rccm.201206-1045OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Schultz ES, Hallberg J, Bellander T, et al. : Early-Life Exposure to Traffic-related Air Pollution and Lung Function in Adolescence. Am J Respir Crit Care Med. 2016;193(2):171–7. 10.1164/rccm.201505-0928OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Gehring U, Gruzieva O, Agius RM, et al. : Air pollution exposure and lung function in children: the ESCAPE project. Environ Health Perspect. 2013;121(11–12):1357–64. 10.1289/ehp.1306770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morales E, Garcia-Esteban R, de la Cruz OA, et al. : Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax. 2015;70(1):64–73. 10.1136/thoraxjnl-2014-205413 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Schultz ES, Hallberg J, Gustafsson PM, et al. : Early life exposure to traffic-related air pollution and lung function in adolescence assessed with impulse oscillometry. J Allergy Clin Immunol. 2016;138(3):930–932.e5. 10.1016/j.jaci.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 36. Gehring U, Wijga AH, Hoek G, et al. : Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3(12):933–42. 10.1016/S2213-2600(15)00426-9 [DOI] [PubMed] [Google Scholar]
- 37. Gref A, Merid SK, Gruzieva O, et al. : Genome-wide Interaction Analysis of Air Pollution Exposure and Childhood Asthma with Functional Follow-up. Am J Respir Crit Care Med. 2017;195(10):1373–1383. 10.1164/rccm.201605-1026OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gauderman WJ, Avol E, Gilliland F, et al. : The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–67. 10.1056/NEJMoa040610 [DOI] [PubMed] [Google Scholar]
- 39. Gauderman WJ, Vora H, McConnell R, et al. : Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369(9561):571–7. 10.1016/S0140-6736(07)60037-3 [DOI] [PubMed] [Google Scholar]
- 40. Gauderman WJ, Urman R, Avol E, et al. : Association of improved air quality with lung development in children. N Engl J Med. 2015;372(10):905–13. 10.1056/NEJMoa1414123 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Moshammer H, Hoek G, Luttmann-Gibson H, et al. : Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173(11):1255–63. 10.1164/rccm.200510-1552OC [DOI] [PubMed] [Google Scholar]
- 42. Turner S, Fielding S, Mullane D, et al. : A longitudinal study of lung function from 1 month to 18 years of age. Thorax. 2014;69(11):1015–20. 10.1136/thoraxjnl-2013-204931 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Gibbs K, Collaco JM, McGrath-Morrow SA: Impact of Tobacco Smoke and Nicotine Exposure on Lung Development. Chest. 2016;149(2):552–61. 10.1378/chest.15-1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma S, Chhabra D, Kho AT, et al. : The genomic origins of asthma. Thorax. 2014;69(5):481–7. 10.1136/thoraxjnl-2014-205166 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Rehan VK, Liu J, Naeem E, et al. : Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012;10:129. 10.1186/1741-7015-10-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Joubert BR, Felix JF, Yousefi P, et al. : DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am J Hum Genet. 2016;98(4):680–96. 10.1016/j.ajhg.2016.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pugmire J, Vasquez MM, Zhou M, et al. : Exposure to parental smoking in childhood is associated with persistence of respiratory symptoms into young adult life. J Allergy Clin Immunol. 2014;134(4):962–965.e4. 10.1016/j.jaci.2014.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferrante G, Antona R, Malizia V, et al. : Smoke exposure as a risk factor for asthma in childhood: a review of current evidence. Allergy Asthma Proc. 2014;35(6):454–61. 10.2500/aap.2014.35.3789 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Upton MN, Smith GD, McConnachie A, et al. : Maternal and personal cigarette smoking synergize to increase airflow limitation in adults. Am J Respir Crit Care Med. 2004;169(4):479–87. 10.1164/rccm.200211-1357OC [DOI] [PubMed] [Google Scholar]
- 50. Foreman MG, Zhang L, Murphy J, et al. : Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med. 2011;184(4):414–20. 10.1164/rccm.201011-1928OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beyer D, Mitfessel H, Gillissen A: Maternal smoking promotes chronic obstructive lung disease in the offspring as adults. Eur J Med Res. 2009;14(Suppl 4):27–31. 10.1186/2047-783X-14-S4-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Garcia-Larsen V, Del Giacco SR, Moreira A, et al. : Asthma and dietary intake: an overview of systematic reviews. Allergy. 2016;71(4):433–42. 10.1111/all.12800 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Litonjua AA, Weiss ST: Vitamin D status through the first 10 years of life: A vital piece of the puzzle in asthma inception. J Allergy Clin Immunol. 2017;139(2):459–61. 10.1016/j.jaci.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hart PH, Lucas RM, Walsh JP, et al. : Vitamin D in fetal development: findings from a birth cohort study. Pediatrics. 2015;135(1):e167–73. 10.1542/peds.2014-1860 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Zosky GR, Hart PH, Whitehouse AJ, et al. : Vitamin D deficiency at 16 to 20 weeks’ gestation is associated with impaired lung function and asthma at 6 years of age. Ann Am Thorac Soc. 2014;11(4):571–7. 10.1513/AnnalsATS.201312-423OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Bisgaard H, Stokholm J, Chawes BL, et al. : Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med. 2016;375(26):2530–9. 10.1056/NEJMoa1503734 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. McEvoy CT, Schilling D, Clay N, et al. : Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311(20):2074–82. 10.1001/jama.2014.5217 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Litonjua AA, Carey VJ, Laranjo N, et al. : Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA. 2016;315(4):362–70. 10.1001/jama.2015.18589 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Chawes BL, Bønnelykke K, Stokholm J, et al. : Effect of Vitamin D 3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA. 2016;315(4):353–61. 10.1001/jama.2015.18318 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Twisk JW, Staal BJ, Brinkman MN, et al. : Tracking of lung function parameters and the longitudinal relationship with lifestyle. Eur Respir J. 1998;12(3):627–34. 10.1183/09031936.98.12030627 [DOI] [PubMed] [Google Scholar]
- 61. Berntsen S, Wisløff T, Nafstad P, et al. : Lung function increases with increasing level of physical activity in school children. Pediatr Exerc Sci. 2008;20(4):402–10. 10.1123/pes.20.4.402 [DOI] [PubMed] [Google Scholar]
- 62. Smith MP, von Berg A, Berdel D, et al. : Physical activity is not associated with spirometric indices in lung-healthy German youth. Eur Respir J. 2016;48(2):428–40. 10.1183/13993003.01408-2015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Benck LR, Cuttica MJ, Colangelo LA, et al. : Association Between Cardiorespiratory Fitness and Lung Health from Young Adulthood to Middle Age. Am J Respir Crit Care Med. 2017;195(9):1236–1243. 10.1164/rccm.201610-2089OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Morgan WJ, Stern DA, Sherrill DL, et al. : Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172(10):1253–8. 10.1164/rccm.200504-525OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hallberg J, Thunqvist P, Schultz ES, et al. : Asthma phenotypes and lung function up to 16 years of age-the BAMSE cohort. Allergy. 2015;70(6):667–73. 10.1111/all.12598 [DOI] [PubMed] [Google Scholar]
- 66. Martinez FD, Wright AL, Taussig LM, et al. : Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–8. 10.1056/NEJM199501193320301 [DOI] [PubMed] [Google Scholar]
- 67. Savenije OE, Granell R, Caudri D, et al. : Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. 2011;127(6):1505–12.e14. 10.1016/j.jaci.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 68. Hovland V, Riiser A, Mowinckel P, et al. : The significance of early recurrent wheeze for asthma outcomes in late childhood. Eur Respir J. 2013;41(4):838–45. 10.1183/09031936.00071512 [DOI] [PubMed] [Google Scholar]
- 69. Strunk RC, Weiss ST, Yates KP, et al. : Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol. 2006;118(5):1040–7. 10.1016/j.jaci.2006.07.053 [DOI] [PubMed] [Google Scholar]
- 70. Feldman AS, He Y, Moore ML, et al. : Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191(1):34–44. 10.1164/rccm.201405-0901PP [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Belgrave DC, Buchan I, Bishop C, et al. : Trajectories of lung function during childhood. Am J Respir Crit Care Med. 2014;189(9):1101–9. 10.1164/rccm.201309-1700OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bisgaard H, Jensen SM, Bønnelykke K: Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185(11):1183–9. 10.1164/rccm.201110-1922OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Young S, O’Keeffe PT, Arnott J, et al. : Lung function, airway responsiveness, and respiratory symptoms before and after bronchiolitis. Arch Dis Child. 1995;72(1):16–24. 10.1136/adc.72.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gern JE: Rhinovirus and the initiation of asthma. Curr Opin Allergy Clin Immunol. 2009;9(1):73–8. 10.1097/ACI.0b013e32831f8f1b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wark PA, Johnston SL, Bucchieri F, et al. : Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–47. 10.1084/jem.20041901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. : Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–7. 10.1016/j.jaci.2005.06.024 [DOI] [PubMed] [Google Scholar]
- 77. Castleman WL, Sorkness RL, Lemanske RF, et al. : Neonatal viral bronchiolitis and pneumonia induces bronchiolar hypoplasia and alveolar dysplasia in rats. Lab Invest. 1988;59(3):387–96. [PubMed] [Google Scholar]
- 78. Jartti T, Nieminen R, Vuorinen T, et al. : Short- and long-term efficacy of prednisolone for first acute rhinovirus-induced wheezing episode. J Allergy Clin Immunol. 2015;135(3):691–8.e9. 10.1016/j.jaci.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Bønnelykke K, Vissing NH, Sevelsted A, et al. : Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015;136(1):81–86.e4. 10.1016/j.jaci.2015.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Bønnelykke K, Sleiman P, Nielsen K, et al. : A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46(1):51–5. 10.1038/ng.2830 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Bochkov YA, Watters K, Ashraf S, et al. : Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112(17):5485–90. 10.1073/pnas.1421178112 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Calişkan M, Bochkov YA, Kreiner-Møller E, et al. : Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368(15):1398–407. 10.1056/NEJMoa1211592 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Godfrey MS, Jankowich MD: The Vital Capacity Is Vital: Epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest. 2016;149(1):238–51. 10.1378/chest.15-1045 [DOI] [PubMed] [Google Scholar]
- 84. Mannino DM, Ford ES, Redd SC: Obstructive and restrictive lung disease and functional limitation: data from the Third National Health and Nutrition Examination. J Intern Med. 2003;254(6):540–7. 10.1111/j.1365-2796.2003.01211.x [DOI] [PubMed] [Google Scholar]
- 85. Soriano JB, Miravitlles M, García-Río F, et al. : Spirometrically-defined restrictive ventilatory defect: population variability and individual determinants. Prim Care Respir J. 2012;21(2):187–93. 10.4104/pcrj.2012.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Guerra S, Sherrill DL, Venker C, et al. : Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65(6):499–504. 10.1136/thx.2009.126052 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Burrows B, Knudson RJ, Lebowitz MD: The relationship of childhood respiratory illness to adult obstructive airway disease. Am Rev Respir Dis. 1977;115(5):751–60. 10.1164/arrd.1977.115.5.751 [DOI] [PubMed] [Google Scholar]
- 88. Fletcher C, Peto R: The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–8. 10.1136/bmj.1.6077.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Speizer FE, Tager IB: Epidemiology of chronic mucus hypersecretion and obstructive airways disease. Epidemiol Rev. 1979;1(1):124–42. 10.1093/oxfordjournals.epirev.a036206 [DOI] [PubMed] [Google Scholar]
- 90. Sluiter HJ, Koëter GH, de Monchy JG, et al. : The Dutch hypothesis (chronic non-specific lung disease) revisited. Eur Respir J. 1991;4(4):479–89. [PubMed] [Google Scholar]
- 91. Sears MR, Greene JM, Willan AR, et al. : A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414–22. 10.1056/NEJMoa022363 [DOI] [PubMed] [Google Scholar]
- 92. Phelan PD, Robertson CF, Olinsky A: The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol. 2002;109(2):189–94. 10.1067/mai.2002.120951 [DOI] [PubMed] [Google Scholar]
- 93. Tai A, Tran H, Roberts M, et al. : The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax. 2014;69(9):805–10. 10.1136/thoraxjnl-2013-204815 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Tagiyeva N, Devereux G, Fielding S, et al. : Outcomes of Childhood Asthma and Wheezy Bronchitis. A 50-Year Cohort Study. Am J Respir Crit Care Med. 2016;193(1):23–30. 10.1164/rccm.201505-0870OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Stern DA, Morgan WJ, Wright AL, et al. : Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370(9589):758–64. 10.1016/S0140-6736(07)61379-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Svanes C, Sunyer J, Plana E, et al. : Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65(1):14–20. 10.1136/thx.2008.112136 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Jackson B, Kubzansky LD, Cohen S, et al. : A matter of life and breath: childhood socioeconomic status is related to young adult pulmonary function in the CARDIA study. Int J Epidemiol. 2004;33(2):271–8. 10.1093/ije/dyh003 [DOI] [PubMed] [Google Scholar]
- 98. Voraphani N, Stern DA, Wright AL, et al. : Risk of current asthma among adult smokers with respiratory syncytial virus illnesses in early life. Am J Respir Crit Care Med. 2014;190(4):392–8. 10.1164/rccm.201311-2095OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Svanes Ø, Skorge TD, Johannessen A, et al. : Respiratory Health in Cleaners in Northern Europe: Is Susceptibility Established in Early Life? PLoS One. 2015;10(7):e0131959. 10.1371/journal.pone.0131959 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Dratva J, Zemp E, Dharmage SC, et al. : Early Life Origins of Lung Ageing: Early Life Exposures and Lung Function Decline in Adulthood in Two European Cohorts Aged 28-73 Years. PLoS One. 2016;11(1):e0145127. 10.1371/journal.pone.0145127 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 101. Guerra S, Stern DA, Zhou M, et al. : Combined effects of parental and active smoking on early lung function deficits: a prospective study from birth to age 26 years. Thorax. 2013;68(11):1021–8. 10.1136/thoraxjnl-2013-203538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Guerra S, Halonen M, Vasquez MM, et al. : Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015;3(8):613–20. 10.1016/S2213-2600(15)00196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Guerra S, Vasquez MM, Spangenberg A, et al. : Club cell secretory protein in serum and bronchoalveolar lavage of patients with asthma. J Allergy Clin Immunol. 2016;138(3):932–934.e1. 10.1016/j.jaci.2016.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Levine CR, Gewolb IH, Allen K, et al. : The safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. Pediatr Res. 2005;58(1):15–21. 10.1203/01.PDR.0000156371.89952.35 [DOI] [PubMed] [Google Scholar]
- 105. Laucho-Contreras ME, Polverino F, Tesfaigzi Y, et al. : Club Cell Protein 16 (CC16) Augmentation: A Potential Disease-modifying Approach for Chronic Obstructive Pulmonary Disease (COPD). Expert Opin Ther Targets. 2016;20(7):869–83. 10.1517/14728222.2016.1139084 [DOI] [PMC free article] [PubMed] [Google Scholar]