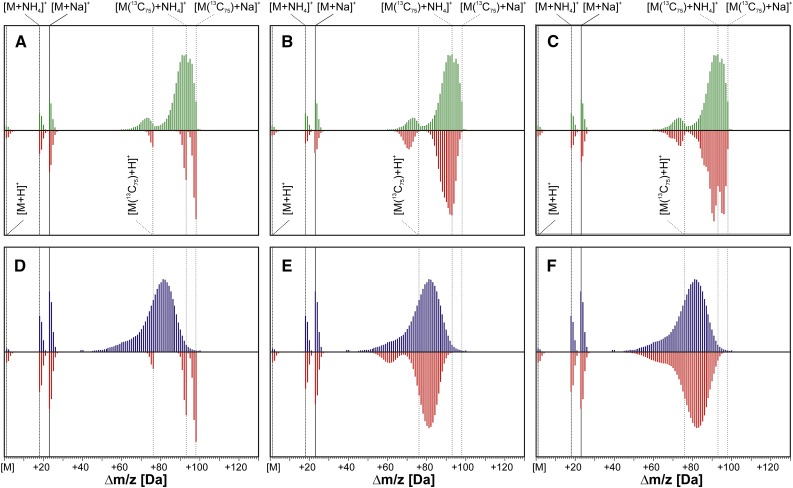
Figure 5.
Comparison of theoretical and experimental mass spectra obtained for labeling of Dol-15 from [U-13C6]Glc in control and osmotic stress conditions. Three variants of the theoretical envelopes were calculated for both control (A–C) and osmotic stress (D–F) conditions. It was assumed that the isotopic enrichment of [13C]Dol-15 was 99% (A and D), that is, it was synthesized from [U-13C6]Glc (99% 13C isotopic abundance) as the sole source of carbon; and was 93.9 ± 1% (B) and 80.7 ± 1.3% (E) to account for some dilution with endogenous native Glc (∼1% 13C isotopic abundance). Alternatively, labeling from [U-13C6]Glc together with an additional involvement of native IPP was considered: 96.1% [U-13C6]Glc and 2.8% native IPP (final isotopic enrichment of Dol-15 was 93.9 ± 1%; C) or 87.4% [U-13C6]Glc and 8.5% native IPP (final isotopic enrichment of Dol-15 was 80.7% ± 1.3%; F). Green and blue traces represent the experimental spectra recorded in control and osmotic stress conditions, respectively, while red traces represent the theoretical ones. Vertical lines indicate the m/z of the cationic, ammoniated ([M+NH4]+ and [M(13C75)+NH4]+), and sodiated ([M+Na]+ and ([M(13C75)+Na]+) forms of the respective monoisotopic ions ([12C75]Dol-15, solid lines; [13C75]Dol-15, dotted lines).