Fig. 6. Mutant obscurin Ig58 exhibits distorted electrostatic topology and target binding characteristics.
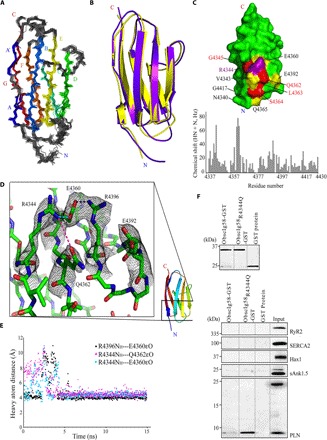
(A) An ensemble of the 20 best NMR structures of wild-type Ig58, colored by β strands A to G. (B) Overlay of the NMR and x-ray structure of wild-type Ig58. (C) Top: Chemical shift differences between mutant and wild-type Ig58 are mapped onto the wild-type Ig58 structure. Residues that exhibit greater than twofold and threefold the average NMR chemical shift change are colored yellow and red, respectively. The location of the R4344Q mutation is colored purple. Bottom: The residue-by-residue chemical shift differences between mutant and wild-type Ig58 HSQC spectra. (D) Electron density of the residues surrounding R4344 (in wild-type Ig58) involved in extensive charge-charge interactions. (E) Molecular dynamics plot of the side-chain N–O distances between surface residues of Ig58, showing long-lived electrostatic interactions. (F) The presence of the R4344Q mutation enhances the ability of Ig58 to interact with PLN. Equivalent amounts of wild-type and mutant glutathione S-transferase (GST)–tagged Ig58 as well as control GST-protein were bound to glutathione matrices (top) and incubated with protein homogenates prepared from adult cardiac muscle. Both wild-type and mutant GST-Ig58 but not control GST-protein efficiently adsorbed the monomeric form of native PLN. Remarkably, mutant GST-Ig58 carrying the R4344Q mutation did so more efficiently than wild-type protein (~10-fold higher). Moreover, neither wild-type nor mutant GST-Ig58 was able to retain other Ca2+-cycling proteins examined, including RyR2, SERCA2, Hax1, and sAnk1.5, further demonstrating the specific interaction between the wild-type and mutant Ig58 domain with PLN.
