ABSTRACT
Blood vessels supply tumor cells with oxygen and nutrients and provide the basis for metastatic dissemination. In addition, endothelial cells can provide factors that orchestrate the behavior of tumor cells. Here, we expand upon our previous findings that link activation of Notch signaling in the endothelium to cellular senescence, weakening of cell junctions, and expression of adhesion molecules, which facilitates tumor and immune cell migration across the vessel wall and homing at distant sites.
KEYWORDS: Angiogenesis, endothelial cell senescence, metastasis, neutrophils, notch signaling
Cancer is a complex disease. Transformed cells act in concert with stromal cells, in particular immune cells, fibroblasts, and endothelial cells. Tumor cells often manage to escape the immune attack and further instruct infiltrating immune cells to differentiate toward a phenotype that supports tumor growth. Tumor endothelial cells also play a major role in tumor progression and metastasis. Growth factors, such as Vascular Endothelial Growth Factor (VEGF), derived from both tumor and immune cells activate quiescent endothelial cells, which form new blood vessels. Based on these findings, anti-angiogenic therapy targeting VEGF or its receptors has emerged and is nowadays widely used in combination with classical chemotherapy. Unfortunately, the success of anti-angiogenic therapies is limited, and many initially sensitive patients become resistant.1
Blood vessels have traditionally been considered to be merely passive tubes with the functional purpose of supplying organs, including tumors, with oxygen and nutrients. However, recent literature has pointed out additional perfusion-independent organ-specific functions of endothelial cells. For example, endothelial cells secrete soluble factors, which orchestrate organ development and regeneration, and the differentiation of stem cell in vascular niches. These functions have recently been described as “angiocrine signaling.”2 In tumors, there is increasing evidence that endothelial cells not only serve to deliver oxygen and nutrients, but also provide angiocrine factors that orchestrate tumor progression and metastasis.3
The endothelium plays another crucial role during metastasis, acting as a physical barrier to tumor cells which must, first, cross to enter the blood stream and, second, to leave it at distant sites. Also, immune cells that enter the primary tumor or metastatic foci must transmigrate through the vessels wall.4 In the primary tumor, transmigration is facilitated by tumor-cell-derived factors and cytokines released from immune cells. However, it remains poorly understood how the endothelium at distant sites contributes to the homing of circulating tumor cells. In our recent publication,5 we have described how tumor cells can modulate Notch signaling within endothelial cells in the primary tumor and within the pre-metastatic niche, rendering the endothelium more permissive for tumor cell homing and transmigration.
Notch signaling is a cell-to-cell communication system that plays a very prominent role in stem cell differentiation, organ development, angiogenesis, and oncogenesis. Binding of Notch ligands triggers cleavage of Notch receptors to release the intracellular domain (Notch-ICD) that enters the nucleus to act as a transcription factor.6 Notch-activating mutations are frequently found in cancer cells; however, the roles of Notch signaling in the tumor stroma are less clear. We observed that Notch1 was commonly activated in endothelial cells within different primary tumor entities, in tumor-infiltrated lymph nodes and in metastases. Notably, the abundance of NOTCH1-ICD-positive endothelial cells was greater in tumor samples compared with healthy control tissues. In advanced stage melanoma, the presence of NOTCH1-ICD-positive endothelial cells correlated with shorter progression-free survival.5 Studies with larger patient cohorts will be necessary to determine if endothelial NOTCH1 activation in tumor biopsies could serve as a biomarker to improve stratification of patients.
There is increasing evidence that Notch signaling controls cellular behavior not only during vessel remodeling, but also in resting endothelial cells within established blood vessels. Notch overactivation occurs e.g. in atherosclerotic plaques,7 under inflammatory conditions,8 and hyperglycemic conditions,9 or driven by tumor cells.5 Notch signaling increases the expression of pro-inflammatory cytokines and adhesion molecules in the endothelium. This facilitates the homing and transmigration of CD11b+/Ly6G+ neutrophils and tumor cells, an effect which is further promoted by Notch-induced endothelial cells senescence.5,7 The senescent cells form weaker cell junctions and express senescence-associated secretory proteins to attract immune cells. Importantly, the induction of endothelial cell senescence and Notch activation was not only observed within the primary tumor mass, but also at distant sites. Here, the induction of adhesion molecules like vascular cell adhesion molecule 1 (VCAM1) facilitates the homing of circulating tumor cells (Fig. 1).5 Future studies will have to address the molecular mechanisms underlying how this occurs. Tumor cells might secrete exosomes containing functional Notch ligands or soluble Notch ligands to prepare the pre-metastatic niche.
Figure 1.
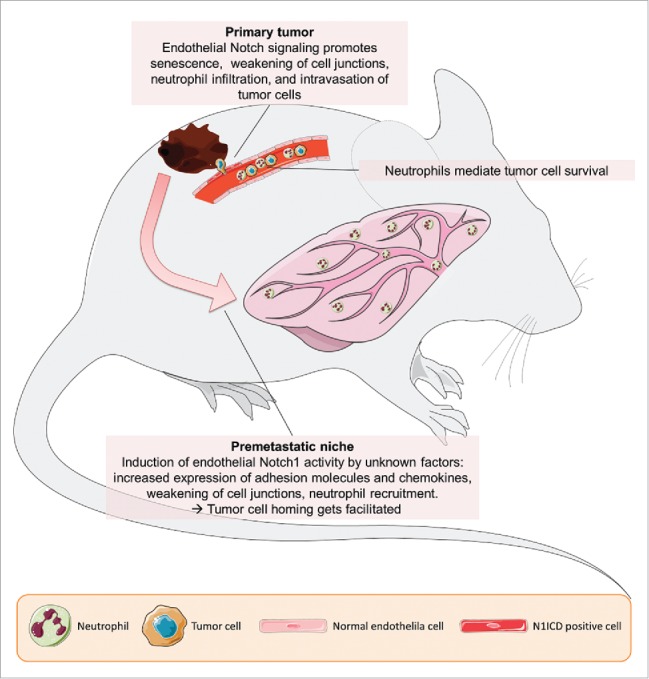
Notch1 signaling in endothelial cells facilitates metastasis. Tumor cells can activate Notch1 on endothelial cells in primary tumor vessels and via unknown mechanisms in vessels in distant sites, such as the lung. This activation triggers several processes within the endothelial cells leading to increased intravasation of tumor cells, and to the conditioning of the premetastatic niche, rendering it less hostile to tumor cells.
In summary, there are many pathological conditions in which Notch signaling plays a detrimental role. Individual components of the Notch signaling pathway have become a recent target for drug development with several Notch-inhibiting drugs currently in phase I/II of clinical trials.10 Ideally, Notch-inhibiting substances could target both oncogenic Notch hyperactivation and angiocrine signaling in tumor endothelial cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008; 8:592-603; PMID:25805408; https://doi.org/ 10.1038/nrc2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature 2016; 529:316-25; PMID:26791722; https://doi.org/ 10.1038/nature17040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer 2010; 10:138-46; PMID:20094048; https://doi.org/ 10.1038/nrc2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reymond N, D’Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer 2013; 13:858-70; PMID:24263189; https://doi.org/ 10.1038/nrc3628 [DOI] [PubMed] [Google Scholar]
- 5.Wieland E, Rodriguez-Vita J, Liebler SS, Mogler C, Moll I, Herberich SE, Espinet E, Herpel E, Menuchin A, Chang-Claude J, et al.. Endothelial Notch1 activity facilitates metastasis. Cancer Cell 2017; 31:355-67; PMID:28238683; https://doi.org/ 10.1016/j.ccell.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 6.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011; 146:873-87; PMID:21925313; https://doi.org/ 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- 7.Liu ZJ, Tan Y, Beecham GW, Seo DM, Tian R, Li Y, Vazquez-Padron RI, Pericak-Vance M, Vance JM, Goldschmidt-Clermont PJ, et al.. Notch activation induces endothelial cell senescence and pro-inflammatory response: Implication of Notch signaling in atherosclerosis. Atherosclerosis 2012; 2:296-303; PMID:23078884; https://doi.org/ 10.1016/j.atherosclerosis.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verginelli F, Adesso L, Limon I, Alisi A, Gueguen M, Panera N, Giorda E, Raimondi L, Ciarapica R, Campese AF, et al.. Activation of an endothelial Notch1-Jagged1 circuit induces VCAM1 expression, an effect amplified by interleukin-1β;. Oncotarget 2015; 6:43216-29; PMID:26646450; https://doi.org/ 10.18632/oncotarget.6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi P, Kuang S. Notch signaling as a novel regulator of metabolism. Trends Endocrinol Metab 2015; 26:248-55; PMID:25805408; https://doi.org/ 10.1016/j.tem.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling—are we there yet? Nat Rev Drug Discov 2014; 13:357-78; PMID:24781550; https://doi.org/ 10.1038/nrd4252 [DOI] [PubMed] [Google Scholar]


