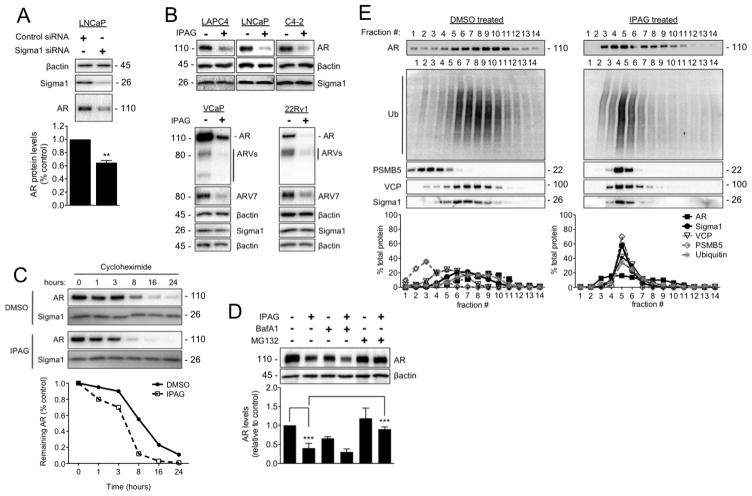
Figure 3. Ubiquitin proteasome-mediated degradation of AR in response to treatment with small molecule Sigma1 inhibitor.
(A) Immunoblot detection of Sigma1 and AR protein levels in LNCaP cells wherein Sigma1 was knocked down by siRNA. Quantification of AR protein in these cells is shown. (B) Immunoblots of AR, AR splice variants (ARV), ARV7, and Sigma1 protein levels following 16 hour treatment of the 5 indicated prostate cancer cell lines with DMSO (−) or 10 μM IPAG (+). (C) AR protein levels in LNCaP cells following treatment with cycloheximide (CHX) for indicated times. (D) Representative immunoblot and quantification of 3 independent immunoblots of AR protein levels in LNCaP cells co-treated for 16 hours with 10 μM IPAG and 1 μM MG132 or 100 nM Bafilomycin A1 (BafA1). (E) Immunoblot of 14 fractions collected following isopycnic fractionation of post-nuclear cell components from LNCaP cells treated for 3 hours with DMSO or 10 μM IPAG. Immunoblots (IB) of AR, p97/VCP (VCP), polyubiquitinated proteins (Ub), 26S proteasome subunit (PSMB5), and Sigma1. Histograms show quantification of immunoblots where the percentage of each protein per fraction is depicted relative to its total across all 14 fractions. All data are presented as mean ± S.E.M. **P < 0.01; ***P < 0.001.