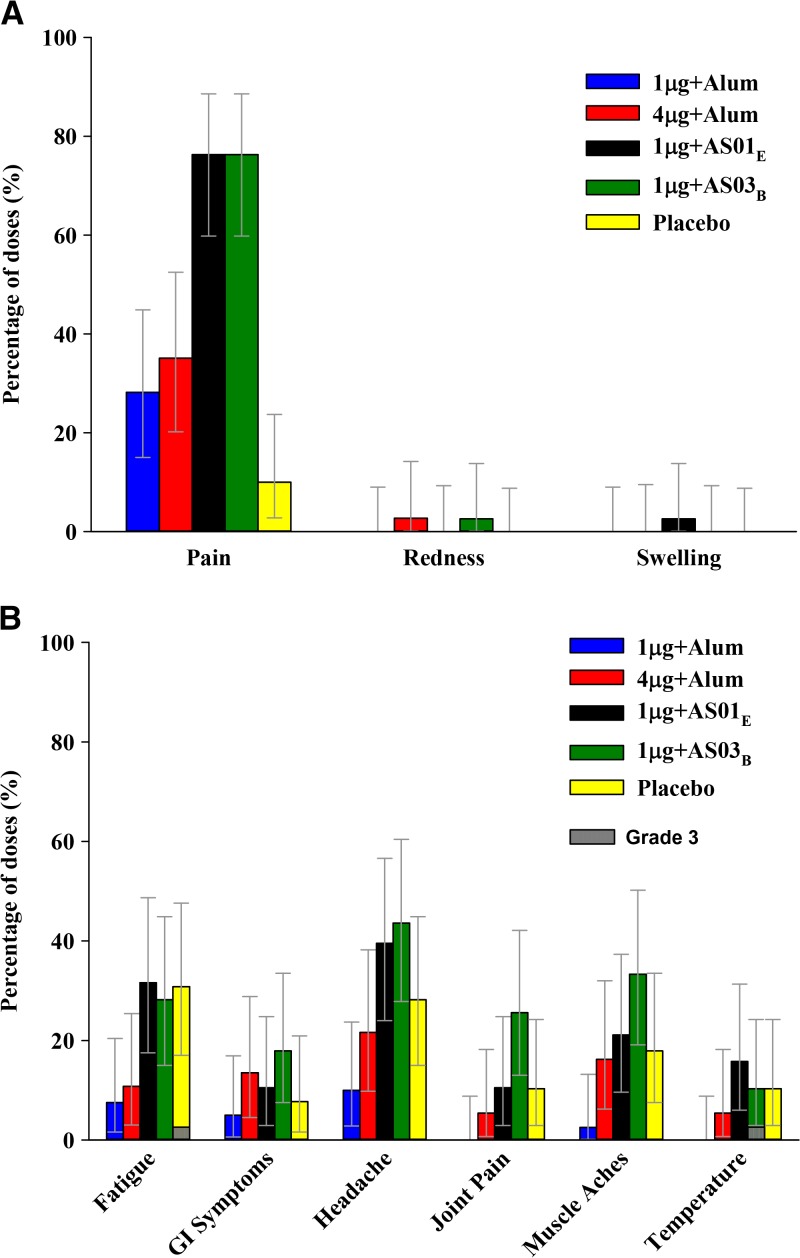
Figure 2.
Overall per dose incidence of any grade solicited injection site (A) and general (B) adverse events during the 7-day postvaccination period (total vaccinated cohort). 1 μg + alum indicates participants who received 1 μg/serotype/dose adjuvanted with alum; 4 μg + alum indicates participants who received 4 μg/serotype/dose adjuvanted with alum; 1 μg + AS01E indicates participants who received 1 μg/serotype/dose adjuvanted with AS01E; 1 μg + AS03B indicates participants who received 1 μg/serotype/dose adjuvanted with AS03B. Error bars indicate exact 95% confidence intervals; GI = gastrointestinal; Temperature = oral temperature ≥ 37.5°C. Intensities of each AE were scored as grades 1–3, with grade 3 fever defined as an oral body temperature ≥ 39°C, grade 3 redness and swelling defined as ≥ 101-mm diameter around the injection site, and all other grade 3 AEs defined as those events preventing normal daily activity.