Figure 1.
Selectively Activating Melanopsin in Space and Time
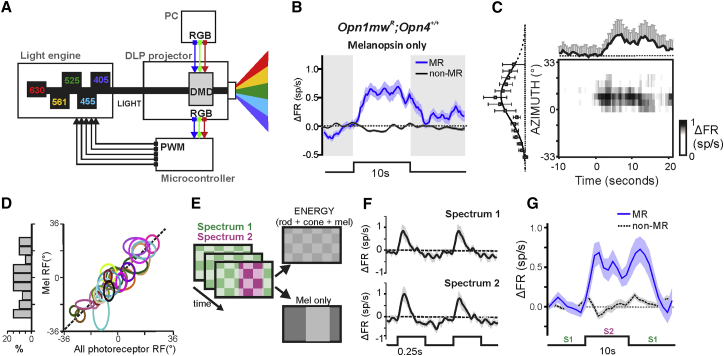
(A) Spectrally controlled stimuli were generated with a DMD projector in which the intrinsic light source was replaced with a five-primary light engine (LEDs with peak emissions: 405 nm, 455 nm, 525 nm, 630 nm, and a 561 nm laser). To control the light engine, the signal normally sent internally to the projector LEDs is rerouted toward the Chipkit Uno 32 microcontroller. Each color plane of an image (red, green, or blue) is separated in time and synchronized with the PWM control of five LEDs, allowing any combination of the five primaries to be separated in space.
(B) Blue line and shading shows mean ± SEM baseline subtracted firing rate (spikes/s) over time of 166 units (out of 668 light response units recorded in dLGN of 25 mice) showing a significant change in firing when presented with a large (72° × 57°) “melanopsin-only” stimulus (10-s presentation of spectrum 2 interleaved with 60-s of spectrum 1) i.e., defined as “MR.” Black line and shading shows mean ± SEM % change in firing rate of 502 units showing no significant change, termed “non-MR.” Timing of stimulus shown below as step and as an interruption of shading on the main plot, dotted line shows baseline activity.
(C) Change in firing rate of a representative MR unit as a function of location on the azimuth (at 4.5° resolution) of a 13° “melanopsin-only” bar presented for 10 s. Main plot shows change in firing over time as a heatmap (scale to right) with mean ± SEM firing at the bar position evoking maximal response shown above. The mean ± SEM change in firing rate (at the time of maximum response for optimal bar) as a function of bar location shown to left, Gaussian curve fit (R2 = 0.86).
(D) Left: histogram of RF centers mapped on azimuth with “melanopsin-only” stimulus. Right: ellipses describing location of RFs on azimuth mapped with “all-photoreceptor” (x axis) and “melanopsin-only” (y axis) stimuli (extent of RF under each condition defined as location on azimuth at half SD on either side of Gaussian fit) for 26 MR units.
(E) A cartoon of stimulus projected to the mouse eye: this stimulus consisted of an inverting checkerboard in which high- and low-radiance squares (7.5° squares inverting at 2 Hz, presenting a 3-fold change in radiance) were high- and low-energy versions of spectrum 1. While maintaining presentation of an inverting checkerboard, a change in the spectrum (from spectrum 1 to spectrum 2) was introduced to a large area of the screen. This stimulus therefore presented two concurrent visual stimuli; a high-frequency inverting checkerboard that was spectrally neutral (i.e., visible to all photoreceptors; top right) and a low-frequency spectral change that was only visible to melanopsin (lower right).
(F) Mean ± SEM responses (baseline subtracted double plot) of MR-units to high-frequency checkerboard inversions rendered in either spectrum 1 (top) or spectrum 2 (bottom). Firing rates are aligned so that checkerboard inversion evoking maximum response is at time 0. Responses were statistically indistinguishable (Paired t test of response amplitude; p = 0.33).
(G) Mean ± SEM firing rate of MR-units (n = 21; black solid line) and non-MR units (n = 81; black dotted line) in response to a transition from spectrum 1 to spectrum 2 (“melanopsin-only”), with superimposed concurrent low-contrast checkerboard inversions.
See also Figures S1 and S2.