Abstract
Standing postural control is complex, meaning that it is dependent upon numerous inputs interacting across multiple temporal-spatial scales. Diminished physiologic complexity of postural sway has been linked to reduced ability to adapt to stressors. We hypothesized that older adults with lower postural sway complexity would experience more falls in the future. 738 adults aged ≥70 years completed the Short Physical Performance Battery test (SPPB) test and assessments of single and dual-task standing postural control. Postural sway complexity was quantified using multiscale entropy. Falls were subsequently tracked for 48 months. Negative binomial regression demonstrated that older adults with lower postural sway complexity in both single and dual-task conditions had higher future fall rate (incident rate ratio (IRR) = 0.98, p = 0.02, 95% Confidence Limits (CL) = 0.96–0.99). Notably, participants in the lowest quintile of complexity during dual-task standing suffered 48% more falls during the four-year follow-up as compared to those in the highest quintile (IRR = 1.48, p = 0.01, 95% CL = 1.09–1.99). Conversely, traditional postural sway metrics or SPPB performance did not associate with future falls. As compared to traditional metrics, the degree of multi-scale complexity contained within standing postural sway-particularly during dual task conditions- appears to be a better predictor of future falls in older adults.
Introduction
Older adults commonly fall due to loss of balance when standing1. The task of standing is most often completed as part of a “dual task;” that is, standing while simultaneously performing additional cognitive tasks such as talking, reading or making decisions in daily life1. Such dual tasking often interferes with performance in one or both tasks, especially in older adults2. Numerous attempts have thus been made to identify fall risk by measuring one’s ability to regulate the movement of their body’s center of mass (i.e., postural sway) with respect to its base of support, under both normal and dual task conditions3. Older adults with a history of falls tend to have larger and faster postural sway when standing in either of these conditions, as compared to those who have not fallen in the past4. However, such traditional characterizations of postural sway, which tend to characterize sway motion or structure at a single temporal or spatial scale (e.g., average sway speed or area) do not sensitively predict those older adults who are more likely to fall in the future5–7. For example, in a study of 100 older adults reported by Maki et al.7, baseline spatial characteristics of postural sway, including average sway speed, did not correlate with prospective fall rates in the ensuing twelve-month follow-up period. We contend that the inability of traditional postural sway metrics to predict future falls may be due to the insensitivity of these metrics to the complex nature of the postural control system.
The regulation of one’s standing postural sway requires the integration of numerous sensory inputs, spinal and supraspinal circuits, a host of cognitive functions and the peripheral neuromuscular system, all operating over different time scales8. Consequently, this control system is inherently non-linear. Characteristic of a non-linear system, the relationship between these inputs and related muscular outputs is continuously modulated over time; the system dynamically “re-weights” the relative influence of each type of feedback on postural muscle activation in order to optimize performance in a given situation9, 10. Similarly, the amount of joint torque created by a given muscular contraction is dependent upon joint stiffness, which is also continuously modulated via changes in muscular tone11. As a result of these and other inter-related and ever-changing control strategies, the seemingly spontaneous fluctuations of postural sway are actually “complex,” meaning that they contain a degree of correlated, fractal-like patterns that exhibit self-similar structures across multiple scales of time and/or space12–15. A previous study has demonstrated that the degree of postural sway complexity is largely independent of traditional sway metrics such as average sway speed or area over time16. We therefore hypothesized that as opposed to traditional metrics, those metrics aimed at capturing the physiologic “complexity” of postural sway will more accurately reflect the integrity of the postural control system and thus, better identify those at risk of suffering falls in the future.
In this study, we conducted a secondary analysis of longitudinal data from the population-based MOBILIZE Boston Study17 to determine the relationship between postural sway and future falls in community-dwelling older adults. Here, we chose to quantify the degree of postural sway complexity during single- and dual-task standing using a technique called multiscale entropy (MSE)18. MSE is one of numerous non-linear time-series analytical techniques that have been used to estimate postural sway complexity19. This approach quantifies the degree of re-occurrence of repetitive patterns within sway fluctuations. However, as opposed to traditional entropy analyses that are limited to one single time scale (e.g., approximate entropy or sample entropy), MSE utilizes a “coarse-graining” technique to estimate the degree of entropy contained within the time series across multiple scales of time. We specifically hypothesized that that those with lower MSE-derived complexity of postural sway during single or dual task standing at baseline would suffer more falls in the future.
Results
765 participants completed baseline tests of postural sway and the Short Physical Performance Battery test (SPPB). 738 of these individuals completed 48 consecutive months of falls tracking. Analyses were limited to these 738 participants. Baseline clinical and functional characteristics of fallers (i.e., those who fell at least once in 48 months) and non-fallers in this cohort are listed in Table 1. The self-reported historical falls rate was higher in fallers than non-fallers (p < 0.001), while all other health characteristics were similar between those who did and did not fall during the study period.
Table 1.
Demographics of fallers and non-fallers.
| Total participants | Fallers (n = 460) | Non-fallers (n = 278) | p-value* | |
|---|---|---|---|---|
| Age (years), Mean ± SD | 78.1 ± 5.4 | 78.2 ± 5.5 | 77.9 ± 5.3 | 0.43 |
| Female, n (%) | 470 (64) | 292 (63) | 178 (64) | 0.79 |
| BMI, Mean ± SD | 27.3 ± 5.1 | 27.3 ± 4.9 | 27.4 ± 5.4 | 0.64 |
| Education (years), Mean ± SD | 14.2 ± 3.1 | 15.0 ± 4.8 | 14.6 ± 7.7 | 0.11 |
| Comorbidity, Mean ± SD | 3.0 ± 1.6 | 3.1 ± 1.6 | 2.9 ± 1.6 | 0.32 |
| SPPB, Mean ± SD | 9.3 ± 2.5 | 9.3 ± 2.6 | 9.4 ± 2.4 | 0.48 |
| Historical falls rate | 0.7 ± 1.3 | 0.9 ± 1.5 | 0.3 ± 0.7 | <0.001 |
| Sway speed (mm/s), Mean ± SD | ||||
| ST | 19.1 ± 4.9 | 19.3 ± 4.9 | 18.9 ± 4.8 | 0.39 |
| DT | 21.5 ± 6.9 | 21.7 ± 7.4 | 21.3 ± 6.1 | 0.43 |
| Sway area (mm2/s), Mean ± SD | ||||
| ST | 183.3 ± 140.7 | 191.1 ± 156.3 | 170.5 ± 109.2 | 0.24 |
| DT | 236.4 ± 217.6 | 248.0 ± 240.3 | 217.1 ± 172.2 | 0.29 |
| AP path length (mm), Mean ± SD | ||||
| ST | 447.1 ± 124.6 | 448.9 ± 123.0 | 443.9 ± 127.4 | 0.59 |
| DT | 506.8 ± 179.1 | 509.7 ± 191.0 | 502.1 ± 157.5 | 0.58 |
| Complexity, Mean ± SD | ||||
| ST | 31.2 ± 6.3 | 30.7 ± 6.4 | 31.9 ± 6.2 | 0.007 |
| DT | 31.0 ± 7.5 | 30.4 ± 7.6 | 32.1 ± 7.3 | 0.002 |
*ANOVAs and Chi-Square test (for sex) were used to determine the differences in these characteristics between fallers and non-fallers.
Note: BMI = body mass index; ST = single task standing; DT = dual task standing; AP = anterioposterior.
When comparing the postural sway metrics, fallers exhibited lower postural sway complexity in both ST and DT conditions (p < 0.007) at baseline than non-fallers, while the traditional parameters (i.e., sway speed, sway area and anterioposterior (AP) path length) or SPPB score did not differ between fallers and non-fallers.
Relationship between baseline standing postural sway and future falls
Figure 1 shows the AP COP time-series during ST from a recurrent faller (A) and a non-faller (B), along with the MSE curves generated from each time-series (C). As compared to the non-faller, the sample entropy of the current faller was lower across multiple scales of time. As such, postural sway complexity (i.e., the area under the MSE curve) of the participant who experienced falls was considerably less than the participant who did not. In contrast, other metrics of postural sway, including sway speed, sway area and AP path length, were quite similar between these two study participants.
Figure 1.
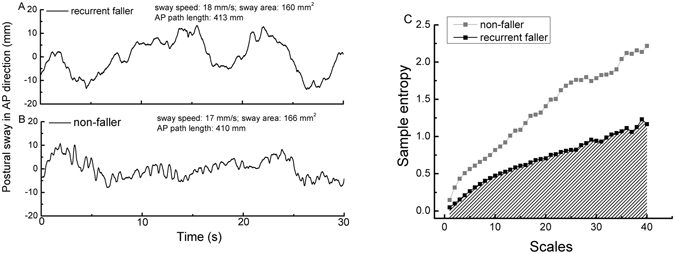
Illustrative anterioposterior (AP) postural sway time-series of a recurrent faller (A) and a non-faller (B) during single task quiet standing along with multiscale entropy (MSE) curves generated from each time-series (C). To quantify the different postural sway dynamics of the time series in A and B, sample entropy was calculated and plotted as a function of time scales (ranging from scale 1 to 40) for each time-series. Postural sway complexity was defined as the area under the multiscale entropy curve, as illustrated by gray shading under the curve of the recurrent faller. When compared to the non-faller, sample entropy of this recurrent faller was lower across multiple time scales. Postural sway complexity (i.e., area under the multiscale entropy curve) of this recurrent faller (complexity = 27.5 units) was nearly half that of the non-faller (complexity = 52.8 units) while other postural sway metrics (i.e., sway speed, area and AP path length) of the two participants were similar (listed on the top of A and B).
The associations between baseline postural sway metrics (i.e., complexity, sway speed, sway area, and AP path length) and the rate of future falls were analyzed using negative binomial regression analyses. Results indicated that the complexity of postural sway during ST (incident rate ratio (IRR) = 0.98, p = 0.02, 5% Confidence Limits (CL): 0.96–0.99) and DT (IRR = 0.98, p = 0.02, 95% CL: 0.97–0.99) was negatively associated with future falls rate (Table 2); one unit difference of either single or dual task postural sway complexity associated with 2% difference in future falls rate. In other words, participants with lower baseline postural sway complexity had a higher rate of future falls. These relationships were independent of age, sex, BMI and historical falls. Conversely, SPPB score or traditional postural sway measures (i.e., sway speed, area, and AP path length) did not significantly predicted the incidence of future falls (IRRs = 1~1.01, p > 0.49).
Table 2.
Relationship between baseline metrics and the rate of future falls#.
| Total falls rate | |||||
|---|---|---|---|---|---|
| IRR | p-value | 95% CL | |||
| Sway speed | ST | 1.01 | 0.49 | 0.99 | 1.03 |
| DT | 1 | 0.87 | 0.99 | 1.02 | |
| Sway area | ST | 1 | 0.92 | 1 | 1.001 |
| DT | 1 | 0.82 | 1 | 1.001 | |
| AP path length | ST | 1 | 0.94 | 1 | 1.001 |
| DT | 1 | 0.89 | 1 | 1.002 | |
| SPPB | 1.01 | 0.76 | 0.96 | 1.05 | |
| Postural sway complexity | ST | 0.98 | 0.02 | 0.96 | 0.99 |
| DT | 0.98 | 0.02 | 0.97 | 0.99 | |
#The negative binomial regression analyses were adjusted for age, sex, BMI and historical falls rate.
Note: ST = single task standing; DT = dual task standing; AP = anterioposterior; IRR = incident rate ratio; 95% CL: 95% Confidence Limits.
Incident rate ratio of future falls in postural sway complexity quintiles
We also divided the cohort into quintiles of postural sway complexity, separately for both ST and DT conditions (Table 3). Participants in the “low-complexity” quintiles were younger those in than “high-complexity” quintiles (p < 0.001) (Table 4). Other health characteristics were similar between quintiles. The lower quintiles suffered higher rates of falls (p < 0.03). Post-hoc analyses revealed that: 1) older adults in Quintile 1 of complexity during ST had a significantly higher falls rate than those in Quintiles 4 and 5 (p < 0.01); and 2) those in Quintiles 1, 2 and 3 of complexity during DT had higher falls rate than those in Quintiles 4 and 5 (p < 0.04).
Table 3.
Postural sway complexity quintiles.
| ST postural sway complexity | DT postural sway complexity | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |||
| Quintile 1 | 23.1 ± 2.5 | 14.8 | 26.2 | 21.3 ± 2.6 | 12.6 | 24.5 |
| Quintile 2 | 27.6 ± 0.8 | 26.2 | 28.9 | 26.7 ± 1.2 | 24.5 | 28.6 |
| Quintile 3 | 30.6 ± 1.0 | 28.9 | 32.2 | 30.5 ± 1.1 | 28.6 | 32.3 |
| Quintile 4 | 33.9 ± 1.0 | 32.2 | 35.8 | 34.3 ± 1.3 | 32.3 | 37.1 |
| Quintile 5 | 40.7 ± 3.9 | 35.7 | 53.2 | 42.3 ± 4.5 | 37.1 | 59.1 |
Note: ST = single task standing; DT = dual task standing.
Table 4.
Descriptive characteristics of participants in quintiles of postural sway complexity.
| Age (years), Mean ± SD | BMI, Mean ± SD | Female, n (%) | Education (years), Mean ± SD | Comorbidity, Mean ± SD | Falls rate#, Mean ± SD | ||
|---|---|---|---|---|---|---|---|
| Single task standing | Quintile 1 | 77.8 ± 5.6A | 27.6 ± 5.0 | 97 (66) | 14.1 ± 3.5 | 3.0 ± 1.7 | 2.6 ± 3.6A |
| Quintile 2 | 77.0 ± 4.9A | 27.4 ± 4.8 | 99 (67) | 14.6 ± 2.9 | 3.2 ± 1.6 | 2.1 ± 3.0AB | |
| Quintile 3 | 77.9 ± 5.7AB | 26.8 ± 5.2 | 93 (63) | 14.3 ± 2.9 | 3.0 ± 1.6 | 2.2 ± 2.8AB | |
| Quintile 4 | 78.3 ± 5.3AB | 27.4 ± 5.1 | 97 (66) | 14.3 ± 3.2 | 2.9 ± 1.5 | 1.8 ± 2.5B | |
| Quintile 5 | 79.6 ± 5.2C | 27.4 ± 5.5 | 84 (57) | 14.1 ± 2.9 | 3.1 ± 1.6 | 1.7 ± 2.4B | |
| p-value* | 0.001** | 0.69 | 0.39 | 0.79 | 0.65 | 0.03** | |
| Dual task standing | Quintile 1 | 77.6 ± 5.7A | 27.4 ± 4.6 | 98 (67) | 14.1 ± 3.3 | 2.8 ± 1.7 | 2.4 ± 2.9A |
| Quintile 2 | 76.9 ± 5.2AB | 27.2 ± 5.1 | 103 (69) | 14.2 ± 3.1 | 2.9 ± 1.4 | 2.5 ± 3.7AB | |
| Quintile 3 | 77.4 ± 5.1A | 27.5 ± 4.9 | 90 (62) | 14.6 ± 3.1 | 3.2 ± 1.7 | 2.1 ± 2.9AB | |
| Quintile 4 | 78.7 ± 5.4AC | 27.5 ± 5.9 | 92 (62) | 14.3 ± 2.9 | 3.3 ± 1.6 | 1.8 ± 2.5BC | |
| Quintile 5 | 80.2 ± 5.1C | 27.0 ± 5.0 | 87 (59) | 14.2 ± 3.0 | 2.9 ± 1.5 | 1.4 ± 2.0C | |
| p-value* | <0.0001** | 0.94 | 0.37 | 0.65 | 0.07 | 0.009** |
#Falls were tracked for 48 months by using self-reported calendar.
*ANOVAs and Student’s t post-hoc tests were used to determine the differences in these characteristics between quintiles. Within each column in ST and DT conditions, mean values with different superscript letters (A, B, or C) are significantly different from one another as determined by Student’s t post-hoc tests, P < 0.05.Note: BMI = body mass index.
Negative binomial regression analyses further indicated that as compared to the highest quintile of complexity (Quintile 5) derived from the ST condition, older adults in the lowest quintile of complexity (Quintile 1) exhibited a (non-significant) trend towards a higher rate of future falls (Table 5, IRR = 1.30, p = 0.07, 95% CL: 0.97–1.75). Notably, older adults in the lower quintiles of complexity (Quintile 1, 2 and 3) derived from the DT condition, however, experienced 42–48% more falls during the follow-up (Table 5, IRRs = 1.42~1.48, p < 0.03, 95% CLs: 1.04–1.99).
Table 5.
Relationship between postural sway complexity quintiles and the rate of future falls#.
| Complexity in ST | Complexity in DT | |||||||
|---|---|---|---|---|---|---|---|---|
| IRR | p-value | 95% CL | IRR | p-value | 95% CL | |||
| Quintile 1 | 1.30 | 0.07 | 0.97 | 1.75 | 1.48 | 0.01 | 1.09 | 1.99 |
| Quintile 2 | 1.14 | 0.41 | 0.84 | 1.54 | 1.42 | 0.03 | 1.04 | 1.93 |
| Quintile 3 | 1.13 | 0.43 | 0.83 | 1.53 | 1.44 | 0.02 | 1.06 | 1.97 |
| Quintile 4 | 0.92 | 0.57 | 0.67 | 1.25 | 1.34 | 0.06 | 0.98 | 1.83 |
| Quintile 5 | Reference | |||||||
#The negative binomial regression analyses were adjusted for age, sex, BMI and historical falls rate. Quintile 5 was set as the reference quintile.
Note: ST = single task standing; DT = dual task standing; IRR = incident rate ratio; 95% CL: 95% Confidence Limits.
Discussion
Falls are a major health concern for older adults because they often result in fractures, hospitalization, diminished mobility, and even death20, 21. Our results from a large cohort of community-dwelling older adults indicate that the multi-scale physiologic complexity of standing postural sway predicts falls in community-dwelling older adults, even after adjusting for age, BMI, sex, and the historical self-reported falls rate. In general, we observed that older adults with lower baseline complexity of AP postural sway when standing quietly or while performing a cognitive task, demonstrated higher fall rates over the ensuing 48 months. In contrast, commonly-used traditional metrics of postural sway, including sway speed, area and path length, or the physical function test (i.e., SPPB) were not predictive of future falls. Taken together, these results suggest that the MSE-derived complexity of standing postural sway provides unique insight into the complex nature of the postural control system that may be utilized to help identify those older adults who are more likely to fall in the future.
The behavior of a given physiological system is controlled by numerous inputs and regulatory elements that interact with one another on multiple scales of time and space22. The “complexity theory of aging” states that age-related alterations in the quantity and/or quality of these components, as well as to their structural and functional connectivity, reduce system functionality and impair an organism’s ability to adapt to stress. Importantly, mounting evidence across numerous physiological systems suggests that these changes also manifest in a reduction of the complexity contained within the dynamics of the system’s behavior or output under basal or “free-running” conditions23–26.
In line with this theory, lower complexity of standing postural sway has been linked to diminished quantity and/or quality of sensory “input” to the postural control system16. Manor et al.16 demonstrated that older adults with reduced ability to detect light pressures applied to the foot soles, or those with reduced visual acuity, had lower standing postural sway complexity as compared to their age-matched counterparts with intact sensory function. Moreover, those older adults with deficits in both sensory systems exhibited even lower sway complexity. Separate studies have also linked lower postural sway complexity to diminished capacity to adapt to stressors, as indicated by frailty27 and falls history28. Here, we have provided first-of-its-kind, prospective evidence that lower postural sway complexity at baseline is also independently associated with higher rates of future falls over a four-year follow up period.
In contrast to postural sway complexity, traditional metrics related to the average speed or magnitude of sway fluctuations did not predict future falls. The majority of balance-related falls stem from complex, multi-scale interactions between the individual, the environment, and the tasks being completed29. As such, traditional postural sway metrics, which reflect the dynamics or behavior of standing postural control on only a single scale of time, may not fully capture a person’s ability to adapt to the stressors of daily life and ultimately, avoid falls. This notion is supported by Fernie et al.5, who similarly reported that average standing postural sway speed did not correlate with the frequency of future falls in 205 older adults aged 80 years and above. This inability of traditional sway metrics to predict falls may stem from the idea that the dynamic characteristics of a healthy postural control states are not simply reflected as less or reduced sway variability30. To this end, Tai Chi training, for example, has been shown to improve mobility in multiple populations, and at the same time, increase the speed of postural sway fluctuations during standing31–33.
In the current study, those who fell during the follow-up period had lower baseline postural sway complexity, within both ST and DT conditions, as compared to those who did not suffer a fall. Interestingly, within the subset of the cohort that fell at least once, we observed that 1) average sway complexity was similar across ST and DT conditions, yet 2) the degree of postural sway complexity specifically derived from DT trials appeared to be a better predictor of the incidence of future falls. Negative binomial regression models demonstrated that participants within the lowest quintile of DT postural sway complexity, as compared to the highest quintile, suffered 48% more falls during the follow-up period. In contrast, there was only a marginal increase in future falls rate in the lowest quintile of ST postural sway complexity as compared to the highest quintile. The particular sensitivity of DT postural sway complexity to future falls may stem from the notion that the DT test condition more closely mimics typical situations in which falls occur; namely, when older adults are standing or walking and attempt to execute concurrent tasks such as speaking to others, reading or problem-solving34–36.
The current observation that those with higher postural sway complexity at baseline have lower incidence of future falls suggests that fall prevention strategies may be optimized by targeting the complex dynamics of postural control. Several studies suggest that loss of complexity is not an obligatory consequence of aging, but instead, can be optimized with appropriate intervention37. These interventions have been aimed at 1) augmenting the quantity and/or quality of a specific input to the postural control system, or 2) simultaneously enhancing multiple functional components of the system37. For example, Zhou et al.38 recently demonstrated that a shoe insole system, delivering sub-sensory vibrations to the foot soles, and thereby increasing the “input” to the postural control system, increased postural sway complexity by an average of 11% in a small cohort of healthy older adults. Moreover, this increase in postural sway complexity significantly correlated with improvement in mobility as measured by Timed-up-and-go test38. On the other hand, Tai Chi training is a multifaceted intervention that enhances multiple elements of the postural control system (e.g., lower extremities strength39, cardiorespiratory fitness40, and cognitive function41). Moreover, it has also been shown to significantly augment the complexity of standing postural sway in both healthy older adults42 and those suffering from peripheral neuropathy43, and reduce the risk of falling44.
In this study, falls were defined as any unintentional event in which the body came to rest on the ground or other lower level. Future work is therefore needed to determine if MSE-derived postural sway complexity can predict more specific types of falls, such as those that occur indoors or result in injury or hospitalization45. As the complexity in the current study is quantified using MSE only in AP postural sway due to the lack of enough signal to noise ratio in the time-series of ML direction, future studies are also needed to examine the functional importance of postural sway complexity within the ML direction. It is also of note that our current results may seem inconsistent with previous studies reporting that biological aging or disease is linked to relatively greater physiologic “complexity”46, 47. Khandoker et al.47, for example, reported that the complexity of minimum foot clearance time series during walking, as quantified using approximate entropy48 on a single time scale, was higher in fallers as compared to non-fallers. We know that physiological systems, including the postural control system, are regulated by numerous functional components that act across multiple tempo-spatial scales. As such, for a given physiologic time-series, estimates of “complexity” derived from single scales may be disparate from those derived and averaged across multiple scales. Costa et al.49 reported that the single-scale entropy (Scale 1) of heart beat time-series was significantly lower in healthy individuals as compared to those with atrial fibrillation (AF). On the other hand, when entropy was averaged across multiple scales, it was much lower in those with AF as compared to their healthy counterparts. This suggests that the dynamics of physiologic systems are dependent upon the measured temporal or spatial scale. Future research should therefore apply and compare these and other analytical techniques (e.g., detrended fluctuation analysis50) to more fully characterized postural sway dynamics. Nevertheless, the present observations indicate that multiscale complexity of standing postural sway, particularly under DT conditions, may aid in the clinical prediction of future falls in older adults. Moreover, fall-prevention strategies specifically designed to restore and enhance physiological complexity may be particularly beneficial within this population.
Methods
Participants
This secondary analysis was completed using data from the population-based MOBILIZE Boston Study (MBS), which aims to investigate and identify novel risk factors for falls in older adults. A complete description of the MBS study has been reported previously17, 51. Briefly, community-dwelling older adults aged 70 years and older who were able to walk 20 feet without personal assistance (walking aids permitted) were included. Those with terminal disease, severe vision or hearing deficits, or diminished cognitive function (i.e., Mini Mental State Examination score ≤18) were excluded. All the experimental methods and protocols were approved by the Hebrew SeniorLife Institutional Review Board (HSL IRB) and carried out in accordance with relevant guidelines. All participants provided written informed consent as approved by the HSL IRB.
At baseline, 765 eligible participants completed a home interview and were assessed for demographic, clinical and functional characteristics, including standing postural control and the Short Physical Performance Battery test (SPPB). Historical falls rate, that is, the self-reported number of falls suffered within the year prior to the baseline assessment, was also recorded.
Falls were then tracked for 48 months using monthly falls calendars and follow-up interviews. Those with incomplete falls tracking data for the entire 48 months (n = 27) were excluded from analyses.
Assessment of standing postural control
Standing postural control was assessed by measuring postural sway (i.e., center-of-pressure, COP) fluctuations at 240 Hz with a force plate (type 9286AA, Kistler, Amherst, NY). Participants stood barefoot with feet shoulder-width apart on the force plate, which was placed with its mediolateral axis parallel to the laboratory wall. Tissue paper was placed on the force plate and chalk outlines of each foot were recorded prior to the first trial. The outline of each participant was then used throughout his/her assessment to ensure consistent foot placement across trials.
Each participant completed five, 30-second trials under two conditions: quiet standing with eyes open (i.e., single task standing, ST) and standing while performing an additional cognitive task (i.e., dual task standing, DT). Trial order was randomized and one minute of rest was given between each trial. In dual task standing trials, participants performed verbalized serial subtractions of three beginning at the number 500. If participants made five or more errors in a single trial, the test was switched to counting backwards by five from 500. In each subsequent trial, participants were asked to continue subtracting from the final number reached in the previous trial.
As previously reported, the signal-to-noise ratio (SNR) of the force plate in the laboratory was examined by comparing the COP signals recorded from a static 50 lb (22.7 kg) weight and a healthy participant26. We found that the SNR of the COP fluctuations in the anterioposterior (AP) direction was larger than 10 while in mediolateral (ML) direction it was smaller than 1. Therefore, we focused the current analysis of postural sway complexity on the COP time series in AP direction only (see Fig. 1).
The complexity metric was computed using MSE. Prior to calculation of MSE, empirical mode decomposition (EMD) was used to remove low-frequency trends and high-frequency noise in the raw time series, which was well-established previously19. Specifically, fluctuations at frequencies over 20 Hz were removed, as they are unlikely to reflect physiologically meaningful control processes. Fluctuations at frequencies less than 0.2 Hz were also removed, so as to ensure that a sufficient number of dynamic patterns occurred within the length of COP time series19.
EMD-filtered time series were “coarse-grained” on different scales of time to capture system dynamics. This procedure divided the time-series into non-overlapping windows of length equaling a scale factor, τ, ranging from 1 to 40 data points in this study. Thus, the coarse-grained series at the largest scale had 180 data points (i.e., 7200 points/40), which meets standard practice for obtaining reliable estimates of sample entropy18. Sample entropy is defined by the negative natural logarithm of the conditional probability that a time-series, having repeated itself within a tolerance r for m points (pattern length), will also repeat itself for m + 1 points without self-matches. The sample entropy of each coarse-grained time-series in this study was computed by choosing m = 2 and r = 15%19. Figure 1C showed the MSE curves generated by plotting sample entropy as a function of time scale from the two COP time-series presented in Fig. 1A and B. Finally, the postural sway complexity metric was identified as the area under the MSE curve (See Fig. 1C, “shaded region”). A larger area reflects higher sample entropy values over multiple time scales and thus, greater complexity.
In addition to postural sway complexity, several traditional measures of postural sway were computed to enable the comparison of their relationship to future falls. These measures included average sway speed (i.e., COP distance traveled in one trial divided by duration of the trial), sway area (i.e., the area of a confidence ellipse enclosing 95% of the COP fluctuation) and AP path length (total COP distance traveled in the AP direction). Since only AP MSE was used, we included AP path length to provide an additional comparison of sway in this specific direction.
Assessment of falls
A fall was defined as unintentionally coming to rest on the ground or other lower level, not as a result of an overwhelming external hazard or a major intrinsic event. Any fall episodes not classified using this definition were reviewed by an adjudication panel52. Falls were tracked for 48 months using monthly post-card calendars. Participants were instructed to record the actual date when they experienced a fall on the calendar and to mail their calendars to the study center at the end of each month. Those who failed to return the calendars were contacted and interviewed by telephone to collect information on their falls. If a fall was reported on the calendar, a structured telephone interview was conducted to clarify the details of the reported falls, including the circumstances and location of the fall, whether the fall caused injury, etc.
Statistical analysis
Analyses were performed with SAS 9.4 and JMP Pro 12 software (SAS Institute, Cary NC). Means, standard deviations (S.D.) and percentages of selected descriptive characteristics were calculated for the study sample. The Chi-Square test was used to identify the difference in dichotomous baseline variables (e.g., sex) and ANOVAs were used to determine the difference in continuous baseline characteristics between fallers and non-fallers.
Negative binomial regression was used to model the association between the baseline postural sway metrics (i.e., complexity, sway speed, sway area and AP path length) during ST or DT conditions, SPPB scores and future fall rates tracked over 48 months. Covariates for all negative binomial models included age, sex, body mass index (BMI) and historical falls rate.
Additionally, we created quintiles of the continuous postural sway complexity metric separately for ST and DT. ANOVAs and Student’s t tests were first performed to determine if selected health characteristics differed between these quintiles (Chi-Square test was used to identify the difference in sex). Negative binomial regression was then used to compare the rate of future falls between different quintiles of postural sway complexity. The quintile of highest postural sway complexity was set as the reference group. Covariates for these models similarly included age, sex, BMI and historical falls rate.
The incident rate ratio (IRR) and 95% confidence intervals (95% CI) were obtained from all the negative binomial models and the significance level for all analyses was set to p < 0.05.
Acknowledgements
This study was supported by grants from the National Institute on Aging to Dr. Lipsitz (AG025037 and AG041785) and Dr. Manor (1-K01-AG044543–01A1). Dr. Lipsitz holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife.
Author Contributions
J.Z., L.L., and B.M., designed the study; I.I. collected the data; J.Z., D.H., L.L., B.M. analyzed the data and performed statistical analyses; J.Z., L.L., and B.M. drafted the manuscript; and all authors contributed to and approved the final version.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Overstall PW, Exton-Smith AN, Imms FJ, Johnson AL. Falls in the elderly related to postural imbalance. Br. Med. J. 1977;6056:261–264. doi: 10.1136/bmj.1.6056.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Springer S, et al. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Movement. Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 3.Shumway-Cook A, Woollacott M, Kerns KA, Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J. Gerontol. A-Bio. 1997;52:M232–40. doi: 10.1093/gerona/52A.4.M232. [DOI] [PubMed] [Google Scholar]
- 4.Melzer I, Benjuya N, Kaplanski J. Postural stability in the elderly: a comparison between fallers and non-fallers. Age. Ageing. 2004;33:602–607. doi: 10.1093/ageing/afh218. [DOI] [PubMed] [Google Scholar]
- 5.Fernie GR, Gryfe CI, Holliday PJ, Llewellyn A. The relationship of postural sway in standing to the incidence of falls in geriatric subjects. Age. Ageing. 1982;11:11–16. doi: 10.1093/ageing/11.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Lajoie Y, Gallagher S. Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch. Gerontol. Geriat. 2004;38:11–26. doi: 10.1016/S0167-4943(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 7.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J. Gerontol. 1994;49:M72–84. doi: 10.1093/geronj/49.2.M72. [DOI] [PubMed] [Google Scholar]
- 8.Alexander NB. Postural control in older adults. J. Am. Geriatr. Soc. 1994;42:93–108. doi: 10.1111/j.1532-5415.1994.tb06081.x. [DOI] [PubMed] [Google Scholar]
- 9.Peterka RJ. Sensorimotor integration in human postural control. J. Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 10.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age. Ageing. 2006;35:ii7–11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 11.Collins JJ, De Luca CJ. The effects of visual input on open-loop and closed-loop postural control mechanisms. Ex. Brain. Res. 1995;103:151–163. doi: 10.1007/BF00241972. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov PC, Amaral LN, Goldberger AL, Stanley HE. Stochastic feedback and the regulation of biological rhythms. Europhys. Lett. 1998;43:363. doi: 10.1209/epl/i1998-00366-3. [DOI] [PubMed] [Google Scholar]
- 13.Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J. Gerontol. A-Bio. 2002;57:B115–125. doi: 10.1093/gerona/57.3.B115. [DOI] [PubMed] [Google Scholar]
- 14.Lipsitz LA. Physiological complexity, aging, and the path to frailty. Sci. Aging. Knowledge. Environ. 2009;16:pe16. doi: 10.1126/sageke.2004.16.pe16. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, et al. The complexity of standing postural control in older adults: a modified detrended fluctuation analysis based upon the empirical mode decomposition algorithm. PLoS. ONE. 2013;8:e62585. doi: 10.1371/journal.pone.0062585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manor B, et al. Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. J. Appl. Physiol. 2010;109:1786–1791. doi: 10.1152/japplphysiol.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leveille SG, et al. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC. Geriatr. 2008;8:1. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa MD, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002;89:068102. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- 19.Gow BJ, Peng CK, Wayne PM, Ahn AC. Multiscale entropy analysis of center-of-pressure dynamics in human postural control: methodological considerations. Entropy. 2015;17:7926–7947. doi: 10.3390/e17127849. [DOI] [Google Scholar]
- 20.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls: a prospective study. JAMA. 1989;261:2663–2668. doi: 10.1001/jama.1989.03420180087036. [DOI] [PubMed] [Google Scholar]
- 21.Province MA, et al. The effects of exercise on falls in elderly patients: a preplanned meta-analysis of the FICSIT trials. JAMA. 1995;273:1341–1347. doi: 10.1001/jama.1995.03520410035023. [DOI] [PubMed] [Google Scholar]
- 22.Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging: potential applications of fractals and chaos theory to senescence. JAMA. 1992;267:1806–1809. doi: 10.1001/jama.1992.03480130122036. [DOI] [PubMed] [Google Scholar]
- 23.Manor B, Lipsitz LA. Physiologic complexity and aging: Implications for physical function and rehabilitation. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;45:287–293. doi: 10.1016/j.pnpbp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pikkujamsa SM, et al. Cardiac interbeat interval dynamics from childhood to senescence comparison of conventional and new measures based on fractals and chaos theory. Circulation. 1999;100:393–299. doi: 10.1161/01.CIR.100.4.393. [DOI] [PubMed] [Google Scholar]
- 25.Yang AC, et al. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: a multiscale entropy analysis. Neurobiol. Aging. 2013;34:428–438. doi: 10.1016/j.neurobiolaging.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan DT, et al. Aging and the complexity of cardiovascular dynamics. Biophys. J. 1991;59:945. doi: 10.1016/S0006-3495(91)82309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang HG, et al. Frailty and the degradation of complex balance dynamics during a dual-task protocol. J. Gerontol. A-Bio. 2009;64:1304–1311. doi: 10.1093/gerona/glp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison S, Colberg SR, Parson HK, Vinik AI. Relation between risk of falling and postural sway complexity in diabetes. Gait. Posture. 2012;35:662–668. doi: 10.1016/j.gaitpost.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. New. Engl. J. Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 30.Stergiou N, Harbourne RT, Cavanaugh JT. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J. Neurol. Phys. Ther. 2006;30:120–119. doi: 10.1097/01.NPT.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- 31.Wolf SL, Barnhart HX, Ellison GL, Coogler CE, Atlanta FICSIT Group The effect of Tai Chi Quan and computerized balance training on postural stability in older subjects. Phys. Ther. 1997;77:371–281. doi: 10.1093/ptj/77.4.371. [DOI] [PubMed] [Google Scholar]
- 32.Mak MK, Ng PL. Mediolateral sway in single-leg stance is the best discriminator of balance performance for Tai-Chi practitioners. Arch. Phys. Med. Rehab. 2003;84:683–686. doi: 10.1016/S0003-9993(02)04810-4. [DOI] [PubMed] [Google Scholar]
- 33.Maki BE, Holliday PJ, Fernie GR. Aging and postural control. J. Am. Geriatr. Soc. 1990;38:1–9. doi: 10.1111/j.1532-5415.1990.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 34.Berg WP, Alessio HM, Mills EM, Tong C. Circumstances and consequences of falls in independent community-dwelling older adults. Age. Ageing. 1997;26:261–268. doi: 10.1093/ageing/26.4.261. [DOI] [PubMed] [Google Scholar]
- 35.Nachreiner NM, Findorff MJ, Wyman JF, McCarthy TC. Circumstances and consequences of falls in community-dwelling older women. J. Womens. Health. 2007;16:1437–1446. doi: 10.1089/jwh.2006.0245. [DOI] [PubMed] [Google Scholar]
- 36.Beauchet O, et al. Recurrent Falls and Dual Task–Related Decrease in Walking Speed: Is There a Relationship? J. Am. Geriatr. Soc. 2008;56:1265–1269. doi: 10.1111/j.1532-5415.2008.01766.x. [DOI] [PubMed] [Google Scholar]
- 37.Manor B, Lipsitz LA. Physiologic complexity and aging: Implications for physical function and rehabilitation. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;45:287–293. doi: 10.1016/j.pnpbp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou. J, Lipsitz LA, Habtemariam D, Manor B. Sub-sensory vibratory noise augments the physiologic complexity of postural control in older adults. J. Neuroeng. Rehabil. 2016;13:44. doi: 10.1186/s12984-016-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong Y, Li JX, Robinson PD. Balance control, flexibility, and cardiorespiratory fitness among older Tai Chi practitioners. Brit. J. Sport. Med. 2000;34:29–34. doi: 10.1136/bjsm.34.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor-Piliae RE, et al. Effects of Tai Chi and Western exercise on physical and cognitive functioning in healthy community-dwelling older adults. J. Aging. Phys. Activ. 2010;18:261–279. doi: 10.1123/japa.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolfson L, et al. Balance and strength training in older adults: intervention gains and Tai Chi maintenance. J. Am. Geriatr. Soc. 1996;44:498–506. doi: 10.1111/j.1532-5415.1996.tb01433.x. [DOI] [PubMed] [Google Scholar]
- 42.Wolf SL, et al. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. J. Am. Geriatr. Soc. 1996;44:489–497. doi: 10.1111/j.1532-5415.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 43.Manor B, Lipsitz LA, Wayne PM, Peng CK, Li L. Complexity-based measures inform tai chi’s impact on standing postural control in older adults with peripheral neuropathy. BMC. Complement. Altern. Med. 2013;13:87. doi: 10.1186/1472-6882-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wayne PM, et al. Complexity-based measures inform effects of Tai Chi training on standing postural control: cross-sectional and randomized trial studies. PLoS. ONE. 2014;9:e114731. doi: 10.1371/journal.pone.0114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelsey JL, et al. Indoor and outdoor falls in older adults are different: the maintenance of balance, independent living, intellect, and Zest in the Elderly of Boston Study. J. Am. Geriatr. Soc. 2010;58:2135–2141. doi: 10.1111/j.1532-5415.2010.03062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol. Aging. 2002;23:1–1. doi: 10.1016/S0197-4580(01)00247-0. [DOI] [PubMed] [Google Scholar]
- 47.Khandoker AH, Palaniswami M, Begg RK. A comparative study on approximate entropy measure and poincaré plot indexes of minimum foot clearance variability in the elderly during walking. J. Neuroeng. Rehabil. 2008;5:4. doi: 10.1186/1743-0003-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pincus SM. Approximate entropy as a measure of system complexity. P. Natl. Acad. Sci. USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys. Rev. E. 2005;71:021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- 50.Goldberger AL, et al. Fractal dynamics in physiology: alterations with disease and aging. P. Natl. Acad. Sci. USA. 2002;99:466–472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samelson EJ, et al. Issues in conducting epidemiologic research among elders: lessons from the MOBILIZE Boston Study. Am. J. Epidemiol. 2008;168:1444–1451. doi: 10.1093/aje/kwn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hannan MT, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. Am. J. Epidemiol. 2010;171:1031–1036. doi: 10.1093/aje/kwq024. [DOI] [PMC free article] [PubMed] [Google Scholar]


