Figure 2.
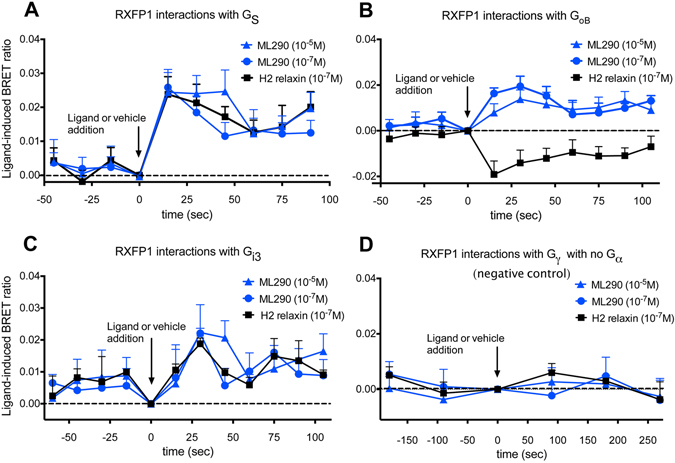
RXFP1 - G protein interactions and treatment with H2 relaxin or ML290. HEK-RXFP1-Rluc8 cells were transiently co-transfected with Gγ2-Venus, Gβ1 and one of Gα subunits (Gαs, GαoB, Gαi3). Interactions between RXFP1 and G proteins were detected prior to and after treatment with H2 relaxin (0.1 μM) or ML290 (0.1 μM or 10 μM) using real-time BRET assays. Both ML290 and H2 relaxin induced interactions between RXFP1-Rluc8 and Gαs, GαoB, and to a lesser extent Gαi3 (A–C). Shifts in BRET ratio between RXFP1 and Gαs were quantitatively and qualitatively similar with ML290 and H2 relaxin. However the ML290 RXFP1- GαoB ligand-induced BRET ratio moved in the opposite direction to that to H2 relaxin (B) suggesting that each ligand induces a different receptor conformation. Interactions between RXFP1 and Gαi3 with H2 relaxin and ML290 were weak but qualitatively similar. There were no interactions between RXFP1-Rluc8 and Gγ2-Venus in the absence of Gα subunits (D). Ligand-induced BRET ratios were calculated by subtracting the BRET ratio for the vehicle-treated sample from that obtained from each ligand-treated sample as described in Materials and Methods. Data are mean ± SEM of 4 independent experiments.
