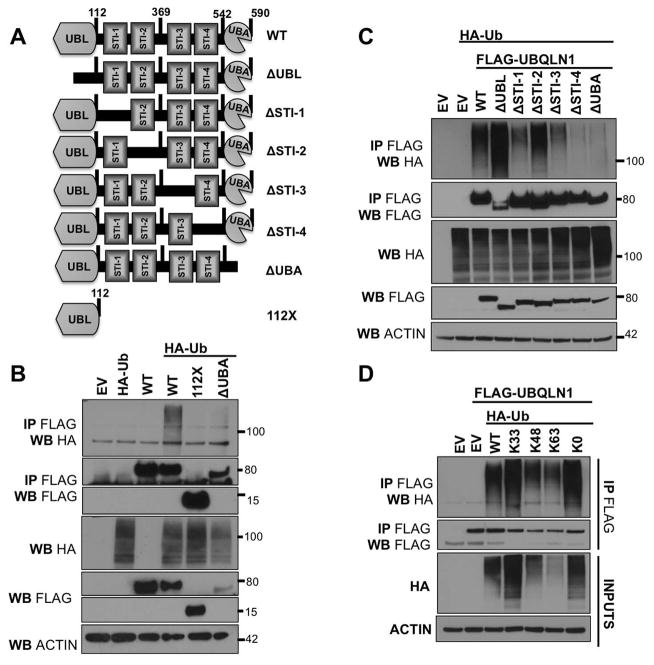
Figure 1. UBQLN1 interacts non-preferentially with diverse ubiquitin linkages through its UBA domain.
(A) Schematic of UBQLN1WT and engineered domain deletion constructs missing individual domains. (B,C), 293T cells were transfected with empty vector (EV), FLAG-UBQLN1WT, domain deletion constructs of UBQLN1 and HA-UbiquitinWT. 48 hours post transfection, cells were lysed and Western Blot analyses were performed with the indicated antibody. Where indicated, immunoprecipitation with anti-FLAG conjugated agarose beads was performed. UBQLN1WT interacts with ubiquitin but UBQLN1112X and UBQLN1ΔUBA (both missing UBA domain) lose this interaction. Therefore, UBQLN1 interacts with ubiquitin through the UBA domain. (D) 293T cells were transfected with FLAG-UBQLN1WT and constructs of HA-UbiquitinWT that have all Lysines (K) mutated to Arginine (R) except one for example, HA-UbiquitinK33 has all K’s are mutated to R except K33 and therefore overexpressing and promoting formation of K33 ubiquitin chains on substrates. K0 indicates all K’s have been mutated to R and this ubiquitin molecule is can only conjugate to the substrate (mono-ubiquitination) and is incapable for forming ubiquitin linkages. UBQLN1 interacts with WT, K33-, K48-, K63- poly-ubiquitin chains and K0 mono-ubiquitinated substrates non-selectively.