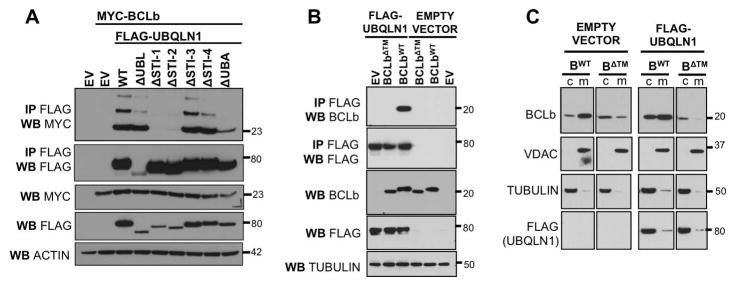
Figure 2. The transmembrane domain of BCLb is recognized by STI-1/2 domains of UBQLN1 and stabilizes BCLb in the cytoplasm.
293T cells were transfected with empty vector (EV), FLAG-UBQLN1WT, or FLAG-tagged domain deletion constructs, MYC-BCLbWT or MYC-BCLbΔTM. 48 hours post transfection, cells were lysed and Western Blot analyses were performed with the indicated antibody. Where indicated, immunoprecipitation with anti-FLAG conjugated agarose beads was performed to determine interaction between UBQLN1 and BCLb proteins. (A) STI-1/2 domains of UBQLN1 are required to interact with BCLb. (B) BCLbΔTM does not interact with UBQLN1. A functional transmembrane domain on BCLb is required for UBQLN1 to recognize and interact with it. (C) 293T cells expressing BCLbWT or BCLbΔTM with EV or FLAG-UBQLN1, were subjected to subcellular fractionation, followed by Western Blot analyses. Cells were fractionated into cytosolic (c) and membranes (m). VDAC1, a membrane bound protein, and Tubulin, a cytoplasmic protein were used to document the validity of the fractionations. UBQLN1 does not interact with BCLbΔTM and therefore does not alter the location of BCLbΔTM, as it is unable to recognize it.