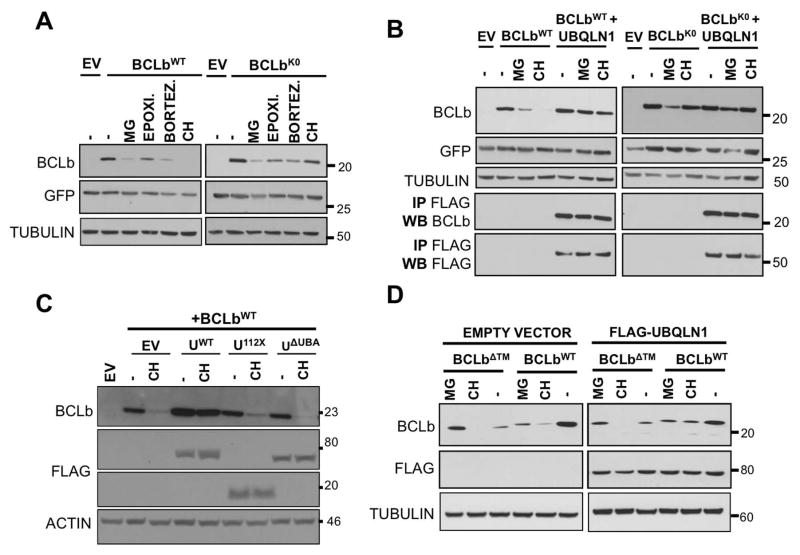
Figure 4. UBA domain of UBQLN1 is responsible for stabilization of BCLbWT.
293T cells were transfected with MIG-BCLbWT, MIG-BCLbWT, MIG-BCLbΔTM, FLAG-UBQLN1WT, domain deletion constructs FLAG-UBQLN1112X and FLAG-UBQLN1ΔUBA as indicated. 36 hours post transfection, cells were treated with the indicated inhibitor for 16 hours; vehicle (−), translational elongation inhibitor Cycloheximide (CH) and proteasomal inhibitors MG132 (MG), Epoximicin (Epoxi.) and Bortezomib (Bortez.). Cells were lysed and Western Blot analyses were performed with the indicated antibody. (A) Stability of BCLbWT and BCLbK0 was tested in the presence of UBQLN1, when exposed to the indicated inhibitors for 16 hours. (B) Experiment was performed as in A but in the presence of UBQLN1. As seen in A, expression of BCLbWT is lost upon exposure to CH for 16 hours, but in the presence of UBQLN1, it is stabilized. Loss of BCLbWT seen with proteasomal inhibitors is also rescued by the presence of UBQLN1. UBQLN1 also prevents loss of BCLbK0 seen with MG132. (C) Experiment was performed as in A in the presence of CH and domain deletion constructs of UBQLN1 and we mapped the UBA domain to be responsible for stabilizing BCLbWT. Both UBQLN112X and UBQLN1ΔUBA failed to prevent loss of BCLbWT. (D) Stability of BCLbΔTM was tested similar to BCLbWT and BCLbK0. BCLbΔTM is generally less stable than BCLbWT and presence of UBQLN1 does not alter its expression.