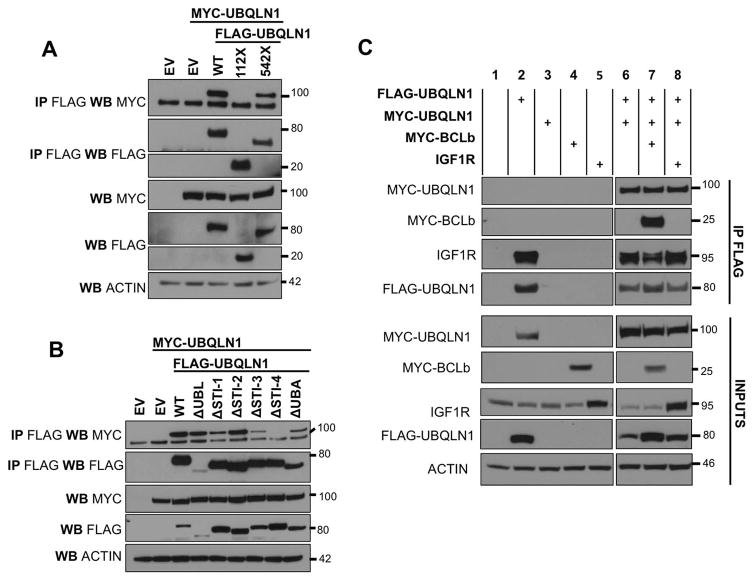
Figure 6. UBQLN1 dimerizes via its STI-4 domains.
293T cells were transfected with empty vector (EV), MYC-UBQLN1WT, FLAG-UBQLN1WT, domain deletion constructs of UBQLN1 MYC-UBQLN1WTas indicated. 48 hours post transfection, cells were lysed and Western Blot analyses were performed with the indicated antibody. Where indicated, immunoprecipitation with anti-FLAG conjugated agarose beads was performed to determine interaction. (A) FLAG-UBQLN1WT interacts with MYC-UBQLN1 and the STI domains located between amino acid 112 and 542 are required for this interaction to take place. (B) Domain deletion constructs map the dimerization to the STI-4 domain. (C) The level of dimerization of UBQLN1 proteins remain unchanged in the presence of endogenous substrates (IGF1R; lanes 2,6,7) and introducing exogenous substrates (BCLb and IGF1R; lanes 7 and 8 respectively).