Abstract
Whereas B7-1/B7-2 and CD28/cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) serve as the main switches regulating the clonal composition of activated naive T cells, other B7 family members fine-tune the expansion and properties of activated T cells. Inducible costimulatory molecule (ICOS)-B7h promotes T-dependent antibody isotype switching and expansion of effector cells. Effector T cells trafficking into inflamed tissues interact with antigen-presenting cells there and are regulated by PD-1 and its ligands. B7-H3 and B7x could control the interaction between effector T cells and the peripheral tissues. The different varieties of regulatory T cells could regulate both naive T cell activation and effector function through costimulatory receptor/ligands.
Keywords: antitumor immunity, autoimmunity, costimulation, inflammation, regulatory T cells
Introduction
The discovery and characterization of new molecules that regulate T cell activities is perhaps one of the most intensely investigated areas in immunology. This is due to the enormous implications and potential of this research toward alleviating many of the scourges of the developed world such as cancer and autoimmune diseases. Two of the most significant developments in recent years have been the great expansion of the number of costimulatory ligands and receptors that belong to the extended B7 and CD28/cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) families of molecules, and the revival of regulatory T cells. Although these topics have been reviewed in detail elsewhere, we would like to propose a framework for the physiological functions of the different B7 family molecules during the distinct phases of an immune response and to integrate this with our increased understanding of regulatory T cells. The main theme is the distinction between the initiation of naive T cell activation and the regulation of effector T cell properties and responses.
In the past decade we have come a long way in terms of levels of complexity from the original two-signal hypothesis [1], which proposed that T cell activation required stimulation both via the T cell receptor (TCR) (signal 1) and through additional costimulatory molecules (signal 2). Instead of a simple binary on/off switch for the initiation of a T cell response, we now understand that costimulation orchestrates the clonal composition and features of the T cell response. Recently, many new costimulatory pathways have been discovered that influence the properties of T cell responses. The discovery of novel costimulatory ligands/receptor pairs has often been followed by a period of uncertainty about whether ligand–receptor engagement is stimulatory or inhibitory. Most initial efforts are designed to distinguish between these two properties, and a period of confusion can, and still does, persist for some time, before a consensus is finally reached. Although the precise functions of the many extended B7 family members remain to be defined, it is clear that they have distinct but also overlapping functions (Fig. 1).
Figure 1.
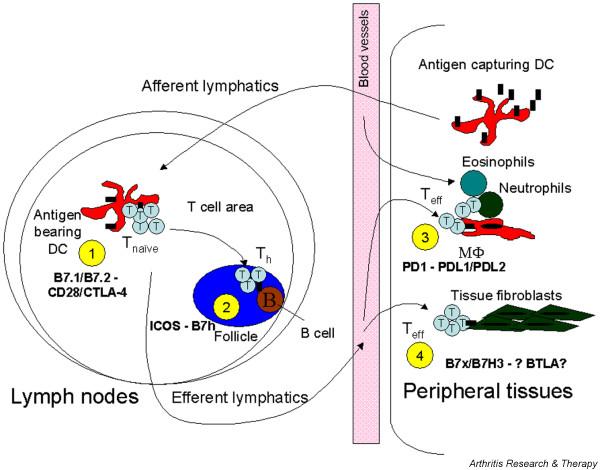
Proposed model for the function of B7 family of costimulatory ligands. 1. B7-1/B7-2 and CD28/cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) regulate the clonal composition of naive T cells that become activated by antigen-bearing dendritic cells (DCs) migrating into the lymphoid organs from the peripheral tissues. 2. After clonal expansion of naive T cells, inducible costimulatory molecule (ICOS)–B7h promotes the T-dependent antibody isotype switching and expansion of effector T cells when the differentiated T helper cells (Th) migrate into the follicles and help to activate germinal-centre B cells. 3. Effector T cells (Teff) trafficking into inflamed tissues interact with antigen-presenting cells such as macrophages and are regulated by programmed death (PD)-1 and its ligands (PDLs). 4. B7-H3 and B7x could be the last-ditch regulators and control the interaction between Teff and the peripheral tissues. BTLA, B and T lymphocyte attenuator.
CD28/CTLA-4: more than just an on/off switch
The CD28/CTLA-4 and B7-1/B7-2 pathway is by far the best-understood costimulatory pathway. Although it has been clear for a while that CD28 helps to initiate T cell responses and CTLA-4 is crucial in the downregulation of responses, our recent studies have focused more on the cell biological lifestyle of these molecules as well as their signaling properties. Much of our understanding of the function of CTLA-4 has been reviewed in detail recently [2]. In brief, the temporal and spatial separation of these two receptors is important in their function. Whereas CTLA-4 has a much higher affinity than CD28 for their ligands, it is not expressed constitutively on naive T cells and is mostly localized intracellularly. After stimulation by the T-cell antigen receptor, CD28 migrates very rapidly into the immunological synapse from the plasma membrane, whereas the intracellular vesicles containing CTLA-4 need to be repositioned to the area of the cytoplasm that is close to the synapse. Once these vesicles have been polarized beneath the T cell–antigen-presenting cell (APC) interface, CTLA-4 can be translocated into the synapse to engage its ligands. We have recently found the preferential recruitment of CTLA-4 into the synapse by B7.1, whereas B7.2 preferentially recruits CD28 [3]. This suggests a previously unrecognized mechanism for tuning the response depending on the relative levels of B7.1/B7.2 expressed on APCs.
Interestingly, the translocation of CTLA-4 into the synapse is proportional to TCR signal strength [4]. Hence, CTLA-4 might differentially restrict the expansion of T cells on the basis of the strength of the TCR signal they receive. Instead of being a simple inhibitor that attenuates T cell responses, CTLA-4 could shape the composition and functional activity (for example T helper 1 [Th1] versus Th2) of the overall pool of T cells with different specificities and affinities, which are activated during the course of an immune response [2,5,6]. Indeed, it has recently been reported that even in the absence of Stat6 (a key signal transducer for interleukin-4 [IL-4]), CTLA-4-deficient T cells can efficiently differentiate into Th2 cells [7]. It was suggested that the increased signal strength of high-affinity T cells that are no longer restricted by CTLA-4 could result in an increased bias towards a Th2 phenotype [7]. However, the issue of whether increased TCR signal leads to Th2 differentiation remains very controversial.
Although the inhibitory effects of CTLA-4 are clear, a variety of endogenous versus exogenous mechanisms have been proposed. Whereas we have focused on understanding the cell-endogenous mechanisms of inhibition [2], others have suggested that CTLA-4 has a role in immunosuppression by CD4+CD25+regulatory T cells (Treg cells; discussed below). It has also been suggested that CTLA-4 has a role in the induction of anergic T cells [8] that could in turn be suppressive [9]. These mechanisms are not necessarily mutually exclusive and might act in concert.
More recently, a splice variant of mouse CTLA-4 was discovered that has a fully intact open reading frame encoding a transmembrane isoform that lacks the B7-1/B7-2-binding domain (liCTLA-4) as a result of skipping exon 2 [10]. There is an association between the autoimmune susceptible strain of NOD mice with a fourfold decrease in the expression of liCTLA-4, which is in turn associated with a silent mutation in exon 2. A ligand-independent isoform for CD28 has also been reported [11]. Future studies will have to reconcile the potential functions of these ligand-independent forms, with our recent findings that ligand binding is required for localizing CTLA-4 to the immunological synapse [3]. Perhaps liCTLA-4 provides a 'tonic' inhibitory signal that decreases the T cell activation threshold during the transient non-specific interactions between T cells and dendritic cells (DCs) that occur continuously in the lymph nodes.
ICOS–B7h: antibody production, effector cell differentiation and function
Inducible costimulatory molecule (ICOS) and B7h were the first extended family members of the CD28/B7 costimulatory receptor–ligand pairs to be discovered after almost a decade. This pair has been the subject of intense study over the past few years [12,13]. The phenotype of B7h-deficient and ICOS-deficient mice clearly indicates that they are a unique receptor–ligand pair that have a positive costimulatory effect. The most striking phenotype of these mice is a defect in T-dependent antibody isotype switching and germinal center formation. CD40 and CD40 ligand (CD40L) could be important in stabilizing the ICOS–B7h interaction between T cells and naive B cells and in promoting germinal center formation [14]. Interestingly, a homozygous mutation of ICOS in human patients leads to an immunodeficiency syndrome characterized by severe reduction in all immunoglobulin subclasses [12]. This is consistent with the hypothesis that the main function of ICOS–B7h is to regulate B cell differentiation, class switching and B cell memory responses through germinal center formation.
Although ICOS was originally perceived to costimulate Th2 responses [15], studies with a variety of infectious pathogens have shown that both Th1 and Th2 cytokines were sometimes (although not consistently) altered [12]. The most consistent findings from studies involving antibody blockade and gene-deficient mice were a decrease in T-dependent antibody isotypes (such as IgG1) and no significant differences in the CD8+ cytotoxic T lymphocyte responses. The ICOS–B7h interaction has also been shown to influence the outcome of pathogenesis in several complex autoimmune diseases, transplants, allergy, and tumor models [12,13]. However, a clear consensus on how and why interfering with ICOS–B7h interactions influences the outcome in these models has not emerged. There is no consistent switch or selective decrease in Th1 versus Th2 cytokines when different systems are compared. A likely explanation is the temporal or kinetic differences between these different experimental models, because adoptive transfer studies have suggested that ICOS–B7h serves to enhance the primary and not the secondary T cell responses in vivo [16,17].
Is there another positive costimulatory receptor for PD-L1 and PD-L2?
Although PD-1 was discovered more than 10 years ago now, it was not until its ligands were cloned and found to be homologous to the B7 family members that it was recognized as a costimulatory molecule. The expression profile of both the ligands [13] and PD-1 would suggest that this interaction is important in regulating effector T cell responses in the peripheral tissues by professional APCs such as DCs, macrophages and also endothelial cells [18-23]. One of the more interesting controversies has been the question of whether PD-L1 (or B7-H1) and PD-L2 (or B7-DC) are costimulatory or inhibitory ligands. Although the autoimmune phenotype of the PD-1-deficient mice clearly suggests an inhibitory function for this receptor [13], evidence has accumulated for an undiscovered second stimulatory receptor. Site-directed point mutations in both PD-L1 and PD-L2 were found to abrogate binding to PD-1, but retained costimulatory activity when expressed as Ig fusion proteins [24]. These mutant Ig-fusion proteins could costimulate both PD-1-/-and wild-type T cells. In addition, two other reports have made the observation that PD–L2-Ig fusion proteins could bind and costimulate PD-1-deficient T cells [25,26].
However, a costimulatory function for PD-L1 would not be consistent with the phenotype reported for the PD-L1-deficient mice [27]. PD-L1-deficient mice accumulate CD8+ T cells in the liver that could cause enhanced autoimmune hepatitis when experimentally challenged, but did not develop spontaneous liver disease [27]. This phenotype is consistent with the observation that PD-L1 is highly expressed on liver Kupffer cells and to a smaller extent on sinusoidal endothelial cells, and its expression can inhibit activated T cells [21]. Although this report implicated an inhibitory role for PD-L1 in the deletion or regulation of CD8+ T cells, dendritic cells from PD-L2-deficient mice have a diminished capacity to activate CD4+ T cells [26]. No other phenotypic effects were described for the PD-L2-deficient animals in this study. The issue of whether PD-L1 and PD-L2 are costimulatory or inhibitory is therefore still unresolved.
On the basis of observations that PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cytokines [20,22,23], we speculated that PD-L1 and PD-L2 might differentially regulate Th1 and Th2 cells [22]. In support of this hypothesis, it has recently been shown that antibody blockade of PD-L2 enhanced the Th2 response in an allergic asthma model [28]. However, reports on PD-L1 blockade do not provide a clear consensus: there have been reports of both positive [29] and negative [18,30] functions of this molecule. Future analysis of the gene-deficient mice, perhaps with infectious disease models that drive Th1 and Th2 responses, should be able to determine whether there is differential regulation of Th1 and Th2 cells by these ligands.
B7-H3 and B7x: last-ditch regulators of the peripheral tissues?
B7-H3 and B7x (also called B7-H4 and B7-S1) are the most recently discovered B7 family members. From our phylogenetic analyses we found that B7-H3 and B7x fall into the same B7 family subgroup. Because they are more similar to each other than to the other B7 familiy members, we have speculated that they might share one or more common receptors. B7-H3 was originally cloned from human DCs [31]. It has a very general mRNA expression (for example heart, kidney, and testes), although the cell types expressing B7-H3 in these tissues remain to be established. The receptor for B7-H3 is still unknown but seems to be rapidly and transiently upregulated on T cells after activation. Although B7-H3 was originally reported to costimulate T cell proliferation, interferon-γ production and Th1 responses, the B7-H3-deficient mice have an enhanced interferon-γ response in airway inflammation experiments, suggesting an inhibitory role [32]. As with PD-L1 and PD-L2, these conflicting observations for B7-H3 should, it is hoped, be resolved by the identification of the co-receptor and detailed studies of the cell biology and signaling properties of these molecules.
We and others have recently identified another member of the B7 family, B7x [33], also called B7-S1 [34] and B7-H4 [35]. In brief, B7x also seems to have a much wider tissue distribution than the original B7-1 and B7-2 molecules, similar to that of B7-H3. It is expressed in several peripheral non-lymphoid tissues including the lung, testis, pancreas, kidney, and liver. It is also expressed in several tumor cell lines. In vitro experiments in our laboratory as in others show that B7x can inhibit proliferation and cytokine production by both CD4 and CD8 T cells [33-35]In vivo, administration of anti-B7x antibodies has been shown to exacerbate experimental autoimmune encephalomyelitis [34]. Taken together, these observations suggest that B7x inhibits T cell responses. However, the complexities that have previously been observed for the PD-1 ligands and for B7-H3 prevent us from completely ruling out the possibility that B7x might be costimulatory under certain conditions. Currently, a candidate for the B7x counter-receptor is B and T lymphocyte attenuator (BTLA) [36], because T cells from BTLA-deficient mice fail to bind B7x-Ig. However, receptor binding assays to prove the pairing of B7x and BTLA formally remain to be performed.
Recently, immunohistological studies have shown that B7x was expressed in most ovarian cancers and in some lung cancer tissue, but not in any melanoma samples [37]. B7x expression was found mainly in cytoplasm and plasma membrane of the lung and ovarian cancer cells themselves. The expression of B7x makes it an attractive potential target for enhancing the anti-tumor immune response, perhaps in conjunction with CTLA-4 blockade. We have already demonstrated the therapeutic potential of CTLA-4 blockade as anti-tumor therapy in human clinical trials [38,39]. PD-L1/B7-H1 has also been proposed to be a good target for boosting anti-tumor immunity [40,41]. Future studies will determine whether B7x is important in tumor immune evasion and would also be a suitable target of anti-tumor immunotherapy.
Costimulation and various regulatory T cells: FOXP3, GITR, and 'anti-suppression'
To understand how the T cell response is coordinated as a whole, it is important to integrate our understanding of 'regulatory' T cells with the emerging concepts in costimulation. At least two different forms of suppressor T cells seem to be recognized at the moment. The first are the so-called 'natural' regulatory CD4+CD25+ (Treg) class because they seem to differentiate from a thymic lineage and are absent from mice that have been thymectomized at an early age [42]. There are significant numbers of these cells in most secondary lymphoid organs where they could prevent the priming of self-reactive naive T cells. Suppressors of the second form are considered to come from an 'induced' type (Tr1), having arisen as a result of priming under specific conditions, instead of being preselected to be suppressors through their TCR [43-45]. The key phenotype of these induced suppressors is the secretion of IL-10 [46], and ICOS is potentially important in the function of these cells [47]. T cells that express high levels of ICOS are often found to co-express IL-10 [48].
The discovery of forkhead box P3 (FOXP3) as a key transcription factor in controlling the differentiation of thymic-lineage-dependent 'natural' CD4+CD25+ Treg cells [49-51] has potentially provided a marker to differentiate between Treg cells and Tr1 cells. However, it is important not to exclude the possibility that FOXP3+ 'natural' Treg cells can also be 'induced' for specific functions under certain conditions. Future work should determine whether these two populations of suppressors can substitute for each other's functions. One interesting possibility is that CD4+CD25+ Treg cells serve primarily to regulate naive T cell priming in the secondary lymphoid organs, whereas Tr1 cells serve to dampen effector T cell responses in the periphery.
With the discovery of multiple layers of immune regulation it is sometimes daunting to consider how an immune response can be triggered at all, even when B7-1 and B7-2 are expressed on dendritic cells. Recently, the emergence of 'anti-suppression' mechanisms has been proposed to explain part of this puzzle. Two forms of anti-suppression have been described so far. The expression of IL-6 by DCs activated through Toll-like receptors has been shown to make responder T cells refractory to suppression by suppressive T cells [52]. In contrast, the recently discovered interaction between glucocorticoid-induced tumor necrosis factor receptor (GITR) and its ligand, GITRL, is thought to abrogate suppression by turning off the ability of suppressor T cells to perform their function [53-55], although this is controversial because GITR is also expressed on recently activated T cells. Antibodies against GITR have been suggested to reverse suppression by CD4+CD25+ cells; they seem to activate signaling into the CD4+CD25+ cells and can shut down their function [53]. The addition of recombinant GITRL has the same effect of reversing suppression [55]. Although the GITR-deficient mice have enhanced T cell responses, they are viable and fertile with no reported signs of autoimmunity, perhaps because of an increased sensitivity to activation-induced cell death. Future work should establish how physiologically important these anti-suppression mechanisms are in controlling the activation of naive T cells in vivo.
When ligands become receptors: the induction of indoleamine 2,3-dioxygenase (IDO) by Treg cells expressing CTLA-4
The linkage between regulatory T cells and costimulation has also come from interesting reports suggesting that some of the B7 family of costimulatory ligands can serve as receptors and transduce signals that change the behaviour of APCs. A naturally occurring human IgM antibody was found to crosslink PD-L2 and to increase antigen presentation and IL-12 production by DCs [56]. After treatment with this antibody either in vitro or in vivo, there was increased DC trafficking to the lymph nodes, suggesting that PD-L2 engagement could enhance DC function.
More importantly, a relationship has been proposed to exist between CTLA-4 engagement of B7-1 and B7-2 and the induction of the tryptophan-catabolizing enzyme IDO [57], which has been previously shown to have a key role in regulating fetal tolerance during pregnancy [58]. CTLA4 Ig fusion proteins have been widely used as a reagent to suppress allograft or xenograft rejection in mouse models of cardiac, liver, and islet transplantation [59]. It has recently been suggested that the major mechanism of action for CTLA4 Ig is not necessarily through the blockade of costimulation of T cells but through the induction of IDO production and tryptophan catabolism as a mechanism regulating T cell activation by increasing apoptosis [60]. It was subsequently shown that CD4+CD25+ Treg cells could induce IDO upregulation and tryptophan catabolism in dendritic cells through a B7-1/B7-2-dependent pathway [57], perhaps as a result of increased surface expression of CTLA-4. The conclusions from these experiments on mice were supported by experiments in vitro with human cells showing similar results [61]. Although these studies are interesting, how and why the CD28 engagement of B7-1 and B7-2 does not also induce immune suppression through IDO are important questions to be answered. It also remains difficult to separate in vivo the effects of costimulatory blockade in the T cells with immune suppression through IDO from the APCs.
Conclusions
We are at very different stages in our understanding of the various costimulatory molecule–ligand pairs. With the original costimulatory ligand pairs of B7-1/B7-2 and CD28/CTLA-4 there is now a fairly detailed biochemical and cell biological understanding of their properties and their physiological functions. The molecular and signaling pathways of the more recently discovered costimulatory receptors such as ICOS and PD-1 have only just begun to be examined, although we are beginning to understand their in vivo functions through the analysis of gene-deficient mice and antibody blockade experiments. With the orphan costimulatory ligands (B7-H3 and B7x) and their potential partners (BTLA), we still know very little about their physiological roles or the signaling pathways that they control. Finally, our understanding of how regulatory T cells develop and perform their function is beginning to coincide with our understanding of costimulatory modulation of T cell activation. Future efforts should lead to greater convergence of these two topical subjects.
Currently we favor the view that CD28 and CTLA-4 are the major switches that regulate the early outcome of TCR engagement during naive T cell activation but can also shape the composition and function of the primed T cell pool. After naive T cells have been primed and begin to undergo clonal expansion, the other B7 family members and their receptors serve as 'lenses' to fine-tune the differentiation and function of the activated T cells. ICOS–B7h interaction could be important in amplifying the primary expansion and promoting the differentiation of effector T cells, perhaps Th2 cells and Tr1 cells. But more importantly, ICOS/B7h has a crucial role in stabilizing T–B interactions and for helping T-dependent antibody isotype switching in B cells. Effector T cells that leave the secondary lymphoid organs and penetrate back into the inflamed tissues are further regulated by interactions between PD-1 and its ligands, especially when the T cells interact with professional APCs in these tissues such as inflammatory macrophages, dendritic cells, and possibly endothelial cells.
Although PD-1 is clearly an inhibitory receptor, there is controversy over whether its ligands PD-L1 and PD-L2 are costimulatory or inhibitory. Differential regulation of PD-L1 and L2 by Th1 and Th2 cytokines also suggests differential function in regulating Th1 and Th2 responses in the peripheral tissues by inflammatory APCs. Finally, B7-H3 and B7x could be important in controlling the interactions between effector T cells and non-APCs in the peripheral tissues. Similarly to the distinct properties of the different costimulatory ligands, the different varieties of regulatory T cell could have different roles in coordinating the initiation phase in the secondary lymphoid organs, as opposed to the effector functions of T cells in inflamed tissues. Regulatory molecules such as IL-6 and GITR might reverse the action of Treg cells by making the responder cells no longer responsive to suppression or by shutting off the Treg cells. Finally, the induction of tryptophan catabolism in dendritic cells by Treg cells could represent a novel mechanism of regulation through starvation-induced apoptosis.
The intense efforts towards understanding T cell regulatory molecules over the 20 years since the discovery of the TCR have shaped much of our understanding today regarding the immune system. After such a great amount of research on this one cell type, there seems to be no shortage of new mechanisms to be discovered. Some of the new challenges for this century will be the translation of this knowledge into therapies that can substantially improve human health.
Abbreviations
APC = antigen-presenting cell; BTLA = B and T lymphocyte attenuator; CTLA-4 = cytotoxic T lymphocyte-associated antigen-4; DC = dendritic cell; FOXP3 = forkhead box P3; GITR = glucocorticoid-induced tumor necrosis factor receptor; ICOS = inducible costimulatory molecule; IDO = indoleamine 2,3-dioxygenase; IL = interleukin; TCR = T cell receptor; Th = T helper; Treg = regulatory T.
Competing interests
None declared.
References
- Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7.1 and B7.2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004. [DOI] [PubMed]
- Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/S1074-7613(01)00259-X. [DOI] [PubMed] [Google Scholar]
- Kuhns MS, Epshteyn V, Sobel RA, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates the size, reactivity, and function of a primed pool of CD4+ T cells. Proc Natl Acad Sci USA. 2000;97:12711–12716. doi: 10.1073/pnas.220423597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz AA, Sullivan TJ, Sobel RA, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) limits the expansion of encephalitogenic T cells in experimental autoimmune encephalomyelitis (EAE)-resistant BALB/c mice. Proc Natl Acad Sci USA. 2002;99:3013–3017. doi: 10.1073/pnas.042684699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour-Jordan H, Grogan JL, Tang Q, Auger JA, Locksley RM, Bluestone JA. CTLA-4 regulates the requirement for cytokine-induced signals in TH2 lineage commitment. Nat Immunol. 2003;4:182–188. doi: 10.1038/ni884. [DOI] [PubMed] [Google Scholar]
- Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/S1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro. Science. 1994;264:1587–1589. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Ma Y, Mikolajczak SA, Charles ML, Yoshida T, Yoshida R, Strathdee CA, Litchfield DW, Ochi A. A novel costimulatory signaling in human T lymphocytes by a splice variant of CD28. Blood. 2002;99:2138–2145. doi: 10.1182/blood.V99.6.2138. [DOI] [PubMed] [Google Scholar]
- Grimbacher B, Warnatz K, Peter HH. The immunological synapse for B-cell memory: the role of the ICOS and its ligand for the longevity of humoral immunity. Curr Opin Allergy Clin Immunol. 2003;3:409–419. doi: 10.1097/00130832-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/S0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- Liang L, Porter EM, Sha WC. Constitutive expression of the B7h ligand for inducible costimulator on naive B cells is extinguished after activation by distinct B cell receptor and interleukin 4 receptor-mediated pathways and can be rescued by CD40 signaling. J Exp Med. 2002;196:97–108. doi: 10.1084/jem.20020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/S1074-7613(00)00011-X. [DOI] [PubMed] [Google Scholar]
- Mak TW, Shahinian A, Yoshinaga SK, Wakeham A, Boucher LM, Pintilie M, Duncan G, Gajewska BU, Gronski M, Eriksson U, et al. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat Immunol. 2003;4:765–772. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- Smith KM, Brewer JM, Webb P, Coyle AJ, Gutierrez-Ramos C, Garside P. Inducible costimulatory molecule-B7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, Th1, and Th2 T cells. J Immunol. 2003;170:2310–2315. doi: 10.4049/jimmunol.170.5.2310. [DOI] [PubMed] [Google Scholar]
- Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Gajewski TF, Fu YX, Zheng P, Liu Y. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med. 2003;197:1721–1730. doi: 10.1084/jem.20022089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, Yagita H, Chen L, Powell J, Pardoll D, et al. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-h1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/S1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, Tsushima F, Hoshino T, Aizawa H, Akiba H, et al. B7-DC regulates asthmatic response by an IFN-γ-dependent mechanism. J Immunol. 2004;172:2530–2541. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- Kanai T, Totsuka T, Uraushihara K, Makita S, Nakamura T, Koganei K, Fukushima T, Akiba H, Yagita H, Okumura K, et al. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. J Immunol. 2003;171:4156–4163. doi: 10.4049/jimmunol.171.8.4156. [DOI] [PubMed] [Google Scholar]
- Tsushima F, Iwai H, Otsuki N, Abe M, Hirose S, Yamazaki T, Akiba H, Yagita H, Takahashi Y, Omura K, et al. Preferential contribution of B7-H1 to programmed death-1-mediated regulation of hapten-specific allergic inflammatory responses. Eur J Immunol. 2003;33:2773–2782. doi: 10.1002/eji.200324084. [DOI] [PubMed] [Google Scholar]
- Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/S1074-7613(03)00147-X. [DOI] [PubMed] [Google Scholar]
- Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K, Chen L. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated anti-tumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr Opin Immunol. 2003;15:690–696. doi: 10.1016/j.coi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- Lohning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, Hamelmann E, Kroczek RA. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+ CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4+ CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/S1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Radhakrishnan S, Ciric B, Tamada K, Shin T, Pardoll DM, Chen L, Rodriguez M, Pease LR. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J Exp Med. 2002;196:1393–1398. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. Immunology at the maternal–fetal interface: lessons for T cell tolerance and suppression. Annu Rev Immunol. 2000;18:367–391. doi: 10.1146/annurev.immunol.18.1.367. [DOI] [PubMed] [Google Scholar]
- Wekerle T, Kurtz J, Bigenzahn S, Takeuchi Y, Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol. 2002;14:592–600. doi: 10.1016/S0952-7915(02)00378-3. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]


