FIGURE 4.
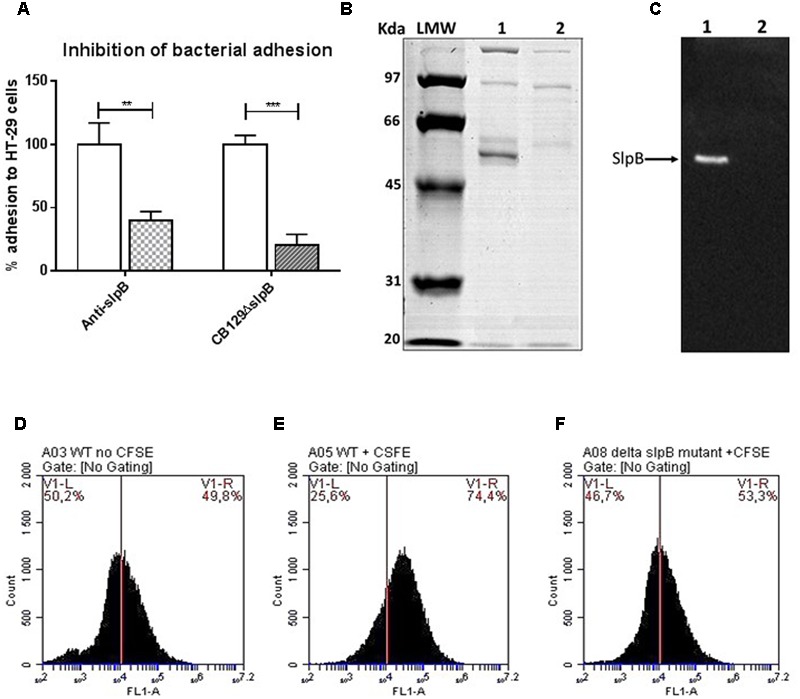
Key role of surface layer protein SlpB in adhesion. (A) Antibodies reduce P. freudenreichii adhesion and so does slpB gene inactivation. Human colon cells were cultured in DMEM prior to co-incubation with propionibacteria. P. freudenreichii CIRM-BIA 129 was treated with antibodies raised against SlpB prior to adhesion assay. As an alternative, the slpB gene was inactivated in P. freudenreichii CIRM-BIA 129 prior to adhesion assay. Adhesion is presented as a percent of the reference CB129 P. freudenreichii strain. (B) Guanidine-extracted surface layer associated proteins were compared by SDS–PAGE in P. freudenreichii CIRM-BIA 129 wild-type (line 1) and in the corresponding mutant CB129ΔslpB (line 2). (C) The corresponding PVDF membrane was subjected to Western Blotting using rabbit antibodies raised against P. freudenreichii surface layer protein SlpB (B). (D,E) Fluorescently labeled live P. freudenreichii CIRM-BIA 129 adheres to cultured human colon epithelial HT-29 cells. The adhesion of CFSE-labeled propionibacteria was detected by the shift in FL1 intensity (E), compared to HT-29 cells with unlabelled propionibacteria (D). Cells (106) were co-incubated with 108 CFU of CFSE-stained propionibacteria for 1 h. At least 50.000 cells per sample were analyzed. As an alternative, labeled P. freudenreichii mutant CB129ΔslpB was co-incubated with HT-29 cells (F). Original gels and western blots, uncropped, are provided in Supplementary Figure S1. Asterisks represent statistically significant differences between strains and were indicated as follows: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Adhesion is presented as a percent of the reference CB129 P. freudenreichii strain.
