Figure 2.
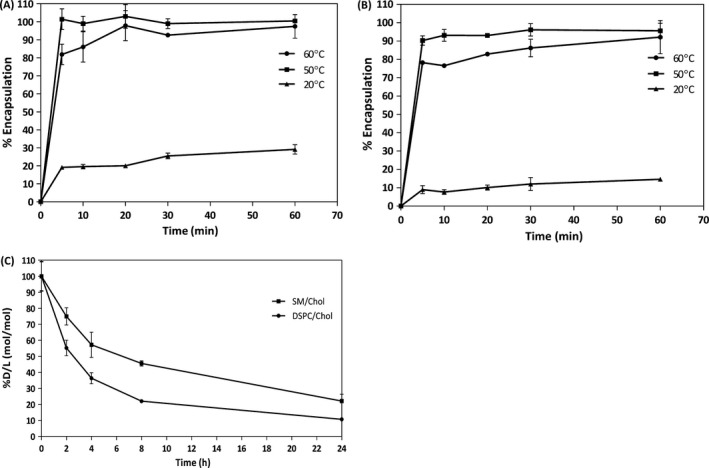
The SM/Chol liposomal topotecan formulation retains topotecan better than DSPC/Chol liposomal topotecan. The liposomes were prepared with unbuffered 300 mmol/L copper sulfate (pH3.5) and the divalent cation ionophore A23187 was added to help maintain the pH gradient following addition of topotecan (0.1 mol topotecan per mole liposomal lipid). Following drug addition, the pH of the solution was immediately adjusted to 7.5. The amount of liposome associated topotecan was determined at the indicated time points as described in the Methods. The results for SM/Chol (panel A) and DSPC/Chol (panel B) liposomes represent the mean ± SD for experiments repeated at least three times. In vitro topotecan release from SM/Chol and DSPC/Chol liposomes (loaded with topotecan using an incubation temperature of 50°C for 30 min) was determined following incubation of the indicated formulation in 80% FBS at 37°C. The amount of retained topotecan (% of initial drug (D) to lipid (L) ratio) was determined over a 24 h time course. Data points represent the mean ± SD for experiments done in duplicate and repeated three times (panel C).
