Figure 3.
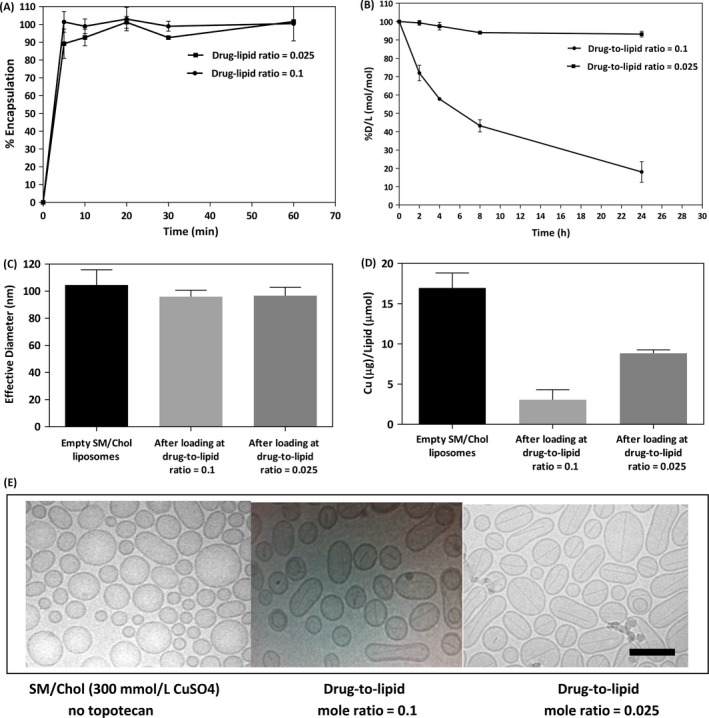
Liposomal topotecan formulations prepared at a 0.025 drug‐to‐lipid mole ratio retained drug better than liposomes prepared at a 0.1 drug‐to‐lipid mole ratio. Topotecan was loaded into SM/Chol liposomes at the indicated drug‐to‐lipid ratio using an incubation temperature of 50°C (panel A). The effect of drug‐to‐lipid ratio on topotecan release from SM/Chol liposomal formulations following incubation in 80% FBS at 37°C over 24 h is shown in Panel B. Decreases in the drug‐to‐lipid ratio represents loss of topotecan from the liposmes over time and each data point represent the mean ± SD of experiments repeated at least three times. The size of SM/Chol liposomes with and without encapsulated topotecan (drug‐to‐lipid mole ratios of 0.1 and 0.025) is shown in panel C; where size was determined using Phase Analysis Light Scattering. The amount of liposome associated copper (μg copper/μmol lipid) before and after topotecan encapsulation (drug‐to‐lipid mole ratios of 0.1 and 0.025) is shown in Panel D, where copper was measured using AAS. Representative cryo‐electron microscopy (CEM) images of SM/Chol liposomes before and after topotecan encapsulation (drug‐to‐lipid mole ratios of 0.1 and 0.025) are shown in panel E. The scale bar is 100 nm.
