Abstract
Electronic cigarettes are advertised as the latest technological gadget—the smoking equivalent of smart phones. I challenge this sense of novelty by tracing their history to the 1960s, when researchers at British American Tobacco first recognized that smokers’ brains were dependent on nicotine. This discovery enabled British American Tobacco to develop a novel kind of smoking device under the codename “Ariel” between 1962 and 1967. Whereas filters were meant to eliminate specific harmful constituents of tobacco smoke, Project Ariel tried to reduce smoking to its alkaloid essence: nicotine. By heating instead of burning tobacco, the scientists working on Ariel managed to produce an aerosol smoking device that delivered nicotine with very little tar while retaining the look and feel of a cigarette. However, after receiving two patents for Ariel, British American Tobacco ultimately decided to abandon the project to avoid endangering cigarettes, its main product. Today, as e-cigarettes are surging in popularity, it is worth revisiting Ariel because it is not just an episode in the history of aerosol smoking devices but its starting point.
Electronic cigarettes have taken the United States by storm and developed into a multibillion dollar industry.1 Although major cigarette producers like Philip Morris (NJoy), R. J. Reynolds (Vuse), and Lorillard (Blu, now belonging to Imperial Tobacco) were initially reluctant to produce e-cigarettes, these companies dominate the e-cigarette market today. However, as e-cigarettes have risen in popularity, it has become clear that even if they are less harmful than cigarettes, their sociological effects are worrisome.2 E-cigarettes are alarmingly popular among minors, who are introduced to nicotine addiction through these widely available devices, and they could lead to a general renormalization of smoking.
Today’s e-cigarettes have well-known precursors in the form of R.J. Reynolds’ Premier (1988) and Eclipse (1996) devices.3 However, by delving into the formerly secret articles of the tobacco industry, we can uncover a much longer prehistory of similar aerosol smoking devices, which were meant to reduce smoking to the administration of nicotine and flavoring. This longer history starts with “Ariel,” a secret project initiated by British American Tobacco (BAT) in 1962.
The goal of Project Ariel was to reimagine what a cigarette could be and develop—its proposal read—
a new smoking device that, by administration of nicotine in a suitable form, should give full satisfaction to smokers while at the same time avoiding the well-known disadvantages inherent in actual smoking.4
The “well-known disadvantages,” of course, were cancer and the host of other diseases caused by tobacco. Whereas filters were intended to eliminate specific harmful constituents of tobacco smoke, Project Ariel tried to reduce smoking to its alkaloid essence: nicotine.
Ariel marks the moment when aerosol smoking devices first became a theoretical and economic possibility—at least in the top secret world of a major tobacco company’s research department. Ariel relied on the results of BAT’s earlier projects “Mad Hatter” and “Hippo,” which found that smokers reached for cigarettes not because they had certain personality traits but because they were addicted to nicotine. Instead of seeing nicotine as a potent poison and mild stimulant (as it used to be understood), BAT researchers now regarded it as the sine qua non of tobacco. This discovery coincided with an exceptional moment in the history of the tobacco industry, when radical redesigns of cigarettes first made sense as a way to circumvent impending cigarette regulation amid the lung cancer crisis of the 1950s. Although Ariel cigarettes were never manufactured on a large scale, the project can tell us about the moment when a tobacco company first grappled with the idea that they were selling not tobacco or cigarettes but an addictive drug: nicotine.
THE ORIGINS OF PROJECT ARIEL
BAT’s interest in alternatives to cigarettes has to be placed within the context of the 1950s, when a series of widely publicized epidemiological studies linked smoking to lung cancer.5 In response, BAT and other tobacco companies mounted a campaign to sow doubt about the connection between smoking and cancer while also promoting their filter cigarettes as safer alternatives to unfiltered cigarettes.
Most tobacco companies initially hoped that filters—some made of asbestos—could selectively eliminate most or all carcinogens.6 However, they soon discovered that selective filters were “a thermodynamic impossibility” (as a Philip Morris executive put it) because all parts of cigarette smoke were harmful.7 This did not stop them, however, from advertising filter cigarettes as healthier because they contained less tar. Filter cigarettes inaugurated a long history of smoking devices that promised “healthier” or “safer” smoke without ever delivering on this promise. In addition to filters, this history includes, for example, “light” and “ultralight” cigarettes, which simply contain less tobacco but are not any healthier because smokers just smoke more of them.8
The same goes for ventilated cigarettes, which have small holes in the filter.9 These holes lower the amount of tar and nicotine measured by automatic smoking machines. Smokers, however, simply cover them with their mouths and absorb much higher doses of tar and nicotine than advertised. Analyses of internal documents behind these supposedly safer or healthier cigarettes have invariably shown that the industry knew that they did not work and sold them to deceive smokers.10 Ariel, however, does not fit this pattern: it was in fact designed to be healthier, and BAT abandoned it partially because it did not live up to its expectations and partially because it allowed one to imagine a world where alternative smoking devices such as Ariel could replace cigarettes.
Sir Charles Ellis drove Ariel’s development in the 1960s. BAT hired Ellis in 1955, when they wanted to expand their research department and looked for a senior scientist to oversee this process.11 When he joined BAT, he was nearing the end of an illustrious career as a physicist.12 Before the Second World War, he had worked in the emerging field of nuclear physics, and in 1940–1941, he was on the Military Application of Uranium Detonation committee, which found that atomic bombs were practically feasible. He was knighted in 1946 for his contributions during the war and worked on the British National Coal Board from 1946 to 1955, overseeing its science department.
MAD HATTER AND HIPPO: 1959–1963
Ellis brought his background in applied science to BAT and advocated a detailed study of tobacco and its principal ingredient, nicotine, through two secret research projects named Mad Hatter and Hippo. These projects showed that smokers were addicted to nicotine, thereby opening the door for a device that would reduce smoking to nicotine administration.
The tobacco industry and independent researchers had long understood that nicotine is the main active alkaloid in tobacco and is responsible for the physiological effects of smoking.13 However, the focus was on nicotine’s effects on the body, not on feelings or behavior, and only a few researchers at the time afforded nicotine a role in explaining why people smoked.14 Instead, the consensus was that individuals with certain personality types were drawn to smoking or that smokers derived oral gratification from cigarettes—an idea that relied heavily on Freudian explanations.15
Project Mad Hatter was a BAT research project conducted under contract by the Battelle Memorial Institute in Geneva, Switzerland, between 1959 and 1962; it advanced a different idea: smokers were drawn to cigarettes because they were addicted to nicotine.16 Ostensibly, Mad Hatter sought to understand how nicotine affects the body, and its official final report was indeed a thorough review of those effects.17 A second, highly confidential report contained a far more controversial finding: nicotine was addictive.18 Initially, the report argued, nicotine allowed smokers to cope with stress through its effects on the endocrine system, namely by stimulating the secretion of corticotropin-releasing factor in the hypothalamus.
After a while, however, smokers developed a tolerance to nicotine that left them with an unbalanced endocrine system when they quit, creating a
crav[ing] for renewed drug intake in order to restore the physiological equilibrium. This unconscious desire explained the addiction of the individual.19
Cravings and withdrawal, the report found, were more than just psychological weaknesses: they had a biological basis in the body’s neuroendocrine system.
Ellis was not deterred by the finding that nicotine was addictive. Rather, he thought that nicotine’s addictiveness had to be weighed against its medical benefits. After all, many other commonly used drugs like barbiturate sleeping pills and opiate painkillers were also addictive.20 Nicotine, Ellis argued, had to be seen as a drug whose advantages far outweighed its disadvantages. He was convinced that “nicotine is a very remarkable beneficent drug that both helps the body to resist external stress and can also show a pronounced tranquilising effect.”21 Indeed, he feared not regulation but the competition from the pharmaceutical industry, whose new “minor tranquilizers,” such as Valium, were sweeping Europe and the United States in the early 1960s.22
To establish how nicotine fared in competition with these new drugs, Ellis initiated Project Hippo, which studied the potential benefits of nicotine and found that it could indeed compete with tranquilizers.23 Nicotine, the researchers claimed, helped the body deal with stress more effectively and controlled weight gain. In particular, Battelle compared nicotine to the tranquilizer reserpine and found that although they had similar tranquilizing properties, nicotine produced fewer side effects.24 The results affirmed Ellis’s conviction that nicotine was indeed a beneficial drug and led him to hope that the tobacco industry was “carrying out an essential and valuable service for the public” by distributing this substance.25
The combined results of Mad Hatter and Hippo were a breakthrough for BAT.26 Mad Hatter established that smokers smoked because they were addicted to nicotine and not because their personalities predisposed them to doing so. It transformed tobacco from a homogenous substance to one with a clearly identifiable, active core—the nicotine—and a carcinogenic remainder. Hippo, in turn, showed that smokers were addicted to nicotine and that nicotine might even be beneficial, useful in the release of stress.
A CIGARETTE FOR THE SPACE AGE
For Ellis, Mad Hatter and Hippo presented an alternative to filter cigarettes. Instead of trying to filter out harmful elements from tobacco smoke, one could reduce tobacco to one key constituent: nicotine. Nicotine is a tricky molecule, and its potential for use in harm reduction is controversial today.27 It is highly toxic and the reason smokers cannot give up cigarettes, making it the deadliest drug in the world; conversely, the evidence that nicotine is harmful in the doses found in cigarettes and nicotine replacement products remains limited. In particular, it is not on its own carcinogenic. Hence, although there are little data on the safety of long-term nicotine use, a recent report by the British Royal College of Physicians concluded,
It is widely accepted that any long-term hazards of nicotine are likely to be of minimal consequence in relation to those associated with continued tobacco use.28
Ellis reasoned that if nicotine held the key to both the pleasure derived from smoking and its grip on the smoker’s behavior then BAT had to develop a device that would separate this pleasurable and addictive molecule from the carcinogenic rest of the tobacco. This device had to meet numerous challenging requirements. Most importantly, it had to administer nicotine without any tar or carbon monoxide; it could use only tobacco as a source of nicotine; and it had to look, feel, and smoke like a normal cigarette.29 Ellis christened this new project “Ariel” after the British Ariel satellite program, which put Britain’s first satellite into orbit in 1962.30
Developing such a product was a formidable task, and Ariel was met by internal opposition at BAT, which Ellis had to overcome. First, during the 1960s, the cigarette industry was still claiming that the link between smoking and lung cancer had not yet been proven. In such an environment, the marketing of an alternative smoking device that would eliminate the carcinogenic tar could easily be read as an admission that cigarettes were indeed dangerous. Second, and more importantly, it was unclear whether it was technically possible to create such a device.31 Ellis defended the project against opposition from his own department by invoking the threat that other companies might try to develop and patent a similar product.32 Assuming future regulation of cigarettes, such a scenario could have left BAT in an untenable position, and the chair’s committee eventually agreed to Ellis’s proposal, stating that possessing patents on such a device would be “a wise insurance” even if it did not deem the project’s chances of success to be high.33
DESIGNING ARIEL: 1962–1965
With funding secured, research on Ariel started in April 1962 under contract at the Battelle Memorial Institute in Geneva. Over the next three years, the main researchers, Herbert Schachner, David Williamson, and Hans Geissbühler, dealt with two separate tasks. First, they had to develop a device that would deliver nicotine—but no other harmful substances—to the smoker. Then, they had to make the inhalation of the nicotine—a toxic and irritating substance—acceptable to the smoker.
By the summer of 1962, Schachner and Williamson were trying to establish a general design for the device that would vaporize nicotine without burning it. Cigarettes usually burn at 700°C–800°C, which is far higher than the 250°C–300°C needed to release the nicotine from tobacco. Schachner and Williamson’s goal was to produce a two-tiered device in which the outer part would provide the heat to liberate the nicotine from the inner part. This could be achieved by developing a device with normal tobacco on the outside and a nicotine extract in an inner tube.34
Burning the outer part would release nicotine from the inner part and allow a smoker to inhale nicotine. The first working model of such a design in September 1962 was a long, thin “Lady” cigarette as the inner part wrapped with aluminum foil and then wrapped with tobacco on the outside.35 It was essentially a cigarette within a cigarette separated by a tube of aluminum.
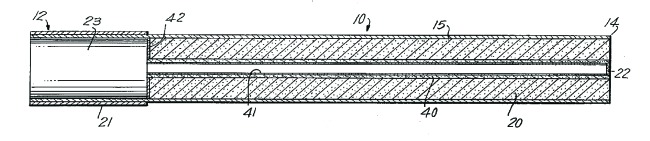
Separating an inner, nicotine-carrying tube from an outer layer of smoldering tobacco was the key design idea, and whereas Battelle tinkered substantially with how this inner tube should be constructed, the basic concept remained.36 The outer tobacco (see Image 2, number 20) burns and heats the ceramic tube (see Image 2, number 40) to about 350°C. At this temperature, the tobacco extract (see Image 2, number 41) coated onto the inner side of the tube releases the nicotine in the form of a vapor. When a smoker draws air in, the nicotine vapor inside the tube cools and becomes an aerosol, providing the sought-after alkaloid to the smoker without combustion products. To ensure that no burnt tobacco enters the smoker’s mouth and lungs, a disk (see Image 2, number 42) blocks the passage from the outer layer. Although this was only one of nine patented designs, this particular one became the basis for Ariel’s future development.37
The second, more challenging, step was the production of an extract that could be placed in the inner tube. The researchers could not use pure nicotine because that would have turned Ariel into a drug-delivery device and, Ellis noted, all but ensured strict regulation.38 Instead, he advocated the development of an extract that “just by good luck” contained a lot of nicotine.39
Of course, creating an extract that delivered nicotine and was acceptable to smokers required engineering ingenuity and not just good luck. First, the extract had to deliver nicotine to the smoker. Because the inner tube could hold only 15 to 50 milligrams of material, Schachner and Williamson experimented with different extraction techniques, which allowed them to produce an extract consisting of 24% nicotine by weight, six times that found in normal tobacco.40 Delivery rates with this extract on standardized smoking machines were meager, however—0.12 to 0.17 milligrams—because the surface area of the extract was too small to permit quick evaporation.41 To remedy this problem, they added activated alumina, which has a very large surface area. This brought the nicotine delivery up to 0.53 to 0.99 milligrams on standardized smoking machines—enough to warrant testing with human participants.42
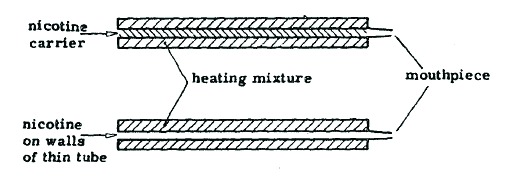
First sketch of two possible Ariel designs from June 1962. In both cases, burning the outer heating mixture would heat up the inner part and liberate nicotine—either from a carrier or coated onto the walls of the tube. The smoker would inhale only the nicotine and not the burning heating mixture.
Source. Herbert Schachner and David Williamson, “Physical Chemical Aspects of Proposed Artificial Cigarettes.” June 1962, Bates no. 301120845-301120869.
Available at: https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=fhmv0194 (accessed April 11, 2017), 12.
Sketch from the first US patent on Ariel from 1966.
Source. Charles Ellis, Herbert Schachner, and David Williamson, US Patent 3 258 015, filed June 28, 1966.
Inhaling the extract, however, was downright dangerous. Battelle’s monthly report from September 1963 stressed that “reactions were so violent and throat irritation was so strong that smoking could not be carried out under regular conditions.”43 Hans Geissbühler, the researcher in charge of this phase of the development, could not explain the reaction. Initially, he thought the problem lay with the taste and tried remedying the situation by adding essential orange oil, but to no avail: “Upon each puff, there was immediate throat irritation which induced coughing.”44 The violent reactions, he discovered in June 1964 by experimenting with a denicotinized extract, were not caused by a replaceable additive but by the one part that could not be replaced: the nicotine itself.45
Administering a sufficient amount of nicotine without inducing throat irritation is a technical challenge on its own and depends on numerous factors, including temperature, particle density, and ratio of molecules in the gaseous to particle phase.46 The key variable, however, is the pH of the smoke. An alkaline smoke means a high amount of unbound, free-base nicotine, which activates receptors in the mouth and the throat and leads to irritation. This is why the alkaline smoke from cigars cannot be inhaled. For Geissbühler, the pH of 7.8 in the aerosol from the Ariel device—compared with 5.0 to 6.0 in normal cigarettes—was clearly a problem. In the summer of 1964 Geissbühler started adding citric acid to the extract, which lowered the pH to 4.4, rendering the aerosol from the extract inhalable.47 With this crucial change, the design of the device and its crucial extract had reached its final form.
PATENTS AND THE END OF ARIEL
Starting off with little more than an ambitious idea, Battelle had managed to produce the first functional aerosol smoking device after 3 years of intensive research between 1962 and 1965. For BAT, this was the moment to transfer Ariel to its own research facility in Southampton to develop the prototype device into a marketable product.48 During the transfer, Ivor Hughes, who replaced Ellis as project leader, wrote a report detailing the successes but also the shortcomings of the device49:
The physiological effect of the nicotine is quite noticeable, although the impact on inhalation tends to be low. The irritation is above normal; the overall flavor of the smoke is not particularly pleasant and is lacking in body.50
Despite these reservations, Hughes also held that the research done so far “shows clearly that the original objective is feasible and achievable.” After all, just three years earlier it had been unclear if a device like Ariel would be feasible at all, so these problems could surely be remedied.51 At this time, the smoke of Ariel as measured by an automatic smoking machine contained 0.8 to 1.0 milligrams of nicotine and 2.0 to 4.0 milligrams baked tar—more than the original goal of delivering no tar but substantially less than filter cigarettes at more than 10.0 milligrams of baked tar.52
To shut out competitors, BAT sought widespread patent coverage for Ariel. Ellis, together with Schachner and Williamson, filed for patents twice: first for numerous different designs in 1964 and then for an improved device with new features in 1965.53 Because Ariel was clearly a response to the lung cancer crisis, the patents were all filed in Battelle’s name to avoid associating BAT with this new device.54
However, after the project was transferred from Battelle to BAT’s own research facility in Southampton in 1965, only a few documents mention Ariel, noting for example that two boxes of functioning Ariel samples had been produced, and no document points to any progress made.55 In July 1967, an internal report noted that the project was dormant for the moment but would probably be reinitiated soon.56 In 1969, it was cancelled altogether.57 By this time, the influence of Ellis, now older than 70 years, at BAT was waning—as was support for the project.58 In contrast to Ellis, Sydney Green, the chief scientist at BAT, thought that aerosol smoking devices like Ariel were unlikely to be commercially successful.59
This lack of commercial viability certainly had to do with the remaining shortcomings of the device described by Hughes. But these shortcomings probably do not suffice to explain why BAT cancelled a still promising program. Rather, BAT may no longer have needed Ariel as an insurance in 1969 because the cigarette industry as a whole found itself in a much improved position. In 1962, when development on Ariel began, BAT had been in a precarious position. New reports about the dangers of smoking continued to pour in. At the same time, the British and the US governments were preparing expert reports that would, as industry executives assumed, indict smoking as the principal cause of lung cancer and form the basis for the future regulation of cigarettes.60
In such a situation, funding an innovative project like Ariel made sense because it could ensure a future for BAT should cigarettes become strictly regulated. In 1969, by contrast, the future for cigarettes looked much brighter. Although both the British report by the Royal College of Physicians in 1962 and the US Surgeon General’s Report in 1964 had indicted smoking, the industry was highly successful in blocking any effective antitobacco regulation.61 Cigarettes were clearly not on their way out. And whatever the future held, the Ariel patents would prevent BAT’s competition from developing similar devices until their expiration date in 1984.
THE LEGACY OF ARIEL
With the end of Ariel, Ellis’s vision of producing a less deadly alternative to cigarettes had been shattered. He retired from BAT in 1972 owing to ill health and died in a nursing home in 1980 at age 84 years.62 In 1988, four years after the Ariel patents expired, R. J. Reynolds introduced “Premier,” an alternative smoking device inspired by Ariel but that burned charcoal instead of tobacco for heat.63 However, poor performance in test markets as well as a National Institute on Drug Abuse report that showed the device could be modified to deliver crack led to the swift end of Premier only one year later.64
In 1994, documents concerning Mad Hatter, Hippo, and Ariel were among the 4000 pages that Merrell Williams, a paralegal working for Brown & Williamson lawyers, had secretly copied and sent to multiple newspapers as well as the tobacco researcher Stan Glantz.65 The publication of these documents caused a sensation, because it demonstrated that the tobacco industry had long known that smoking caused cancer and that nicotine was addictive.66
Quotations from Ellis, for example his description of smoking as a “habit of addiction,” have played an important role in this process: they showed that an industry scientist—Ellis—had understood smoking as an addiction in the 1960s whereas industry executives were still maintaining that smoking was not addictive in the 1990s.67 Access to internal documents highlighted a pervasive pattern of internal knowledge of health risks and organized external denial.68 It also documented that new products like filtered, “slim,” or light cigarettes were never intended to be healthier. Rather, they were designed to trick smokers into thinking they were healthier.69
In Project Ariel, we find a different pattern of secrecy: BAT hid Ariel not because it was fraudulent but precisely because it worked: unlike other tobacco industry gimmicks, such as light cigarettes, the Ariel device was genuinely designed to be healthier and the developed prototypes showed tar deliveries far below those of filter cigarettes. Ellis indeed knew that smoking was an addiction, but he used this knowledge and the conviction that nicotine was a beneficial drug to suggest the development of an alternative, less deadly smoking device that might at the same time make a lot of money for BAT. Of course, as with every other industry innovation, deception was part of Project Ariel. But here the deception served a different purpose: instead of revealing that BAT promoted a defective product, the internal documents show that BAT presumably shut down Ariel precisely because it worked—it was threatening because it permitted one to think of a future when cigarettes could be replaced with a healthier way of administering nicotine.70
Ariel marks a turning point for the tobacco industry. Launched by the discovery that nicotine is addictive, the Ariel device is the physical embodiment of the moment when a major tobacco company first grappled with the notion that it might, after all, be in the business of selling nicotine rather than tobacco or cigarettes. The history of Ariel does not answer current questions regarding e-cigarettes, because it does not show whether smoking harms such as lung cancer and cardiovascular disease can be separated from nicotine addiction. But it does show how these questions themselves first became possible when a new, biological understanding of addiction led to the development of an equally new aerosol smoking device that would deliver nicotine without the usual dose of carcinogens.
In the half century that separates Ariel from e-cigarettes, the devices have matured and are no longer as irritating as Ariel was.71 However, in their understanding of addiction and their technological approach to the smoking problem, they follow the template established half a century ago by BAT through projects Mad Hatter, Hippo, and Ariel.
ACKNOWLEDGMENTS
I would like to thank Robert Proctor for encouraging me to write this article and providing comments on successive drafts. I am also grateful for the suggestions that arose from presenting an early version of this article at the meeting of the History of Science Society; November 9, 2014; Chicago, IL.
ENDNOTES
- 1. Esterl M. “E-Cig Makers Breathe Easier after FDA Proposes Rules; Agency Avoids Heavy-Handed Approach to Regulating Popular Devices,” Wall Street Journal (Online), 2014 Apr 24 2014.
- 2. Rachel Grana, Neal Benowitz, and Stanton A. Glantz, “E-Cigarettes a Scientific Review,” Circulation 129, no. 19 (2014): 1972–1986. [DOI] [PMC free article] [PubMed]
- 3. J. Slade, “Nicotine Delivery Devices,” in Nicotine Addiction: Principles and Management, ed. C. Tracy Orleans and John Slade (New York, NY: Oxford University Press, 1993), 3–23; J. Slade, G. N. Connolly, and D. Lymperis, “Eclipse: Does It Live up to Its Health Claims?” Tob Control 11, suppl 2 (2002): i64–i70. [DOI] [PMC free article] [PubMed]
- 4. Battelle Memorial Institute, “Research Proposal Regarding Project Ariel,” 1962, Bates no. 301120592-301120600. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=hlbx0199 (accessed April 11, 2017), 1.
- 5. A. Brandt, The Cigarette Century: The Rise, Fall, and Deadly Persistence of the Product That Defined America (New York, NY: Basic Books, 2007), ch. 5.
- 6. R. N. Proctor, Golden Holocaust: Origins of the Cigarette Catastrophe and the Case for Abolition (Berkeley, CA: University of California Press, 2011), chs. 16 and 19; Bradford Harris, “The Intractable Cigarette ‘Filter Problem,’” Tob Control 20, suppl 1 (2011): i10–i16. [DOI] [PMC free article] [PubMed]
- 7. A. E. O’Keefe, “Selective Filtration.” 1958, Bates no. 1001902921-1001902924. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/kxww0115 (accessed April 11, 2017), 1.
- 8. Proctor, Golden Holocaust, ch. 22.
- 9. Ibid., ch. 20.
- 10. Ibid., chs. 16, 19, 20, 22.
- 11. British American Tobacco, “Research and Development—Staffing.” 1955, Bates no. 100162933-100162935. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=kxhf0211 (accessed April 11, 2017), 3.
- 12. Kenneth Hutchison, J. A. Gray, and Harrie Massey, “Charles Drummond Ellis. 11 August 1895–10 January 1980,” Biogr Mem Fellows R Soc 27 (1981):199–233.
- 13. American Tobacco, “Pharmacology of Nicotine.” 1940, Bates no. 968301995–968301999. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=jrbp0134 (accessed April 11, 2017)
- 14. Among the few were Lennox M. Johnston, “Tobacco Smoking and Nicotine,” Lancet 240, no. 6225 (1942): 742; J. K. Finnegan, P. S. Larson, and H. B. Haag, “The Role of Nicotine in the Cigarette Habit,” Science 102, no. 2639(1945): 94–96. [DOI] [PubMed]
- 15. British American Tobacco, “Research and Development—Staffing”; Daniel S. P. Schubert, “Personality Implications of Cigarette Smoking Among College Students,” J Consult Psychol 23, no. 4 (1959): 376.
- 16. C. Ellis, “The Effects of Smoking: Proposal for Further Research Contracts with Battelle.” 1962, Bates no. 301083820-301083835. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=pjnc0200 (accessed April 11, 2017), 1–2.
- 17. Geissbühler H, Haselbach C. “The Fate of Nicotine in the Body.” 1 May 1963, Bates no. 1143. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=srgc0040 (accessed April 11, 2017)
- 18. C. Haselbach and O. Libert, “A Tentative Hypothesis on Nicotine Addiction.” 1963, Bates no. 1200–1201. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=qzfh0097 (accessed April 11, 2017)
- 19. Ibid., 3.
- 20. M. Seevers, “Drug Addictions” in Pharmacology in Medicine. A Collaborative Textbook, ed. V. Drill (New York, NY: McGraw-Hill; 1958), 237–252.
- 21. C. Ellis, “Research Conference, Southampton, 1962: Smoking and Health—Policy on Research.” 1962, Bates no. 110070785-110070883. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=sfcp0203 (accessed April 11, 2017), 13.
- 22. Ellis, “Effects of Smoking.”.
- 23. J. Hersch, O. Libert, and C. Rogg-Effront, “Final Report on Project Hippo I.” 1962, Bates no. 105620620–105620683. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=zrml0042 (accessed April 11, 2017). C. Haselbach and O. Libert. “Final Report on Project Hippo II.” 1963, Bates no. 2790. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=jtml0042 (accessed April 11, 2017)
- 24. In hindsight, reserpine and nicotine are hardly suitable for a comparison. Reserpine is a major tranquilizer, a category of drugs used primarily as antipsychotics for severely mentally ill patients, especially schizophrenics. David Healy, The Creation of Psychopharmacology (Cambridge, MA: Harvard University Press, 2002), 102–107. It seems that Battelle selected reserpine because it was one of the most widely used early tranquilizers. O. Libert, “Report No. 1 Regarding Project Hippo II.” 1962, Bates no. 105620684-105620705. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=ysml0042 (accessed April 11, 2017), 2–3.
- 25. C. Ellis, “Re: Research Sponsored at Battelle Memorial Institute.” 1963, Bates no. 105620762–105620764. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=thvw0040 (accessed April 11, 2017)
- 26. When two scientists of the British Tobacco Research Council reviewed the reports, they called the data “poorly presented by any standards” and challenged many of the conclusions. See A. K. Armitage and J. H. Burn, “Appraisal or Reports on Project Hippo 1 (A36) and Project Hippo 2 (A37 & A38).” 1963, Bates no. 689650692-689650700. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=fgdj0191 (accessed April 11, 2017), 1.
- 27. US Department of Health and Human Services, The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Vol. 17 (Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014)
- 28. Royal College of Physicians, Nicotine Without Smoke: Tobacco Harm Reduction (London, UK: Royal College of Physicians; 2016), 58.
- 29. Battelle Memorial Institute, “Research Proposal Regarding Project Ariel.” 1962, Bates no. 100335808–100335816. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=lsdn0208 (accessed April 11, 2017)
- 30. The satellite program was headed up by Harrie Massey, a prewar colleague of Ellis, who would later coauthor Ellis’s biographical memoir. Hutchison et al., “Charles Drummond Ellis,” 1895–1910.
- 31. There was a project to develop an aerosol smoking device at BAT’s US subsidiary Brown & Williamson in 1960. Charles Moll, “Smoker’s Article.” 1960, Bates no. 301120838–301120844. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=mykh0199 (accessed April 11, 2017). However, Ellis cast the idea off as “just a tobacco flavoured nasal spray.” Charles Ellis, “Smoker’s Article: Patent Application by Moll.” 1960, Bates no. 301120837. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=lykh0199 (accessed April 11, 2017)
- 32. Ellis had intelligence that R. J. Reynolds and Philip Morris might be trying to develop an alternative smoking device. “Project Ariel.” 1961, Bates no. 301083852. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=hnlk0203 (accessed April 11, 2017)
- 33. Charles Ellis, “[Note from Ellis Charles Regarding Hippo II and Ariel].” 1962, Bates on. 301083852. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=xmlk0203 (accessed April 11, 2017)
- 34. H. Schachner and D. Williamson, “Physical Chemical Aspects of Proposed Artificial Cigarettes.” 1962, Bates no. 301120845-301120869. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=fhmv0194 (accessed April 11, 2017), 11–13.
- 35. H. Schachner and D. V. S. Williamson, “Physical Aspects of Project Ariel.” 1962, Bates no. 301083696-301083704. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=zxlk0203 (accessed April 11, 2017), 5.
- 36. C. Ellis, H. Schachner, and D. Williamson. Smoking Device. US Patent 3 258 015, filed June 28, 1966.
- 37. Ibid.
- 38. Charles Ellis, “[Note from Charles Ellis to DSF Hobson Regarding Aerosol Smoking Devices].” 1961, Bates on. 301120830–301120831. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=pplf0200 (accessed April 11, 2017)
- 39. Battelle Memorial Institute, “Research Proposal Regarding Project Ariel.”.
- 40. H. Schachner, “Project Ariel. Letter-Report.” 1963, Bates no. 301121873–301121879. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=xzvd0204 (accessed April 11, 2017)
- 41. Ibid., 4.
- 42. Ibid., 4; Battelle Memorial Institute, “Project Ariel. Letter-Report.” 1963, Bates no. 100339502–100339511. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=ttgl0214 (accessed April 11, 2017)
- 43. H. Geissbühler, “Project Ariel. Letter Report,” 1963, Bates no. 301084328. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=znhf0201 (accessed April 11, 2017). Battelle Memorial Institute, “Project Ariel. Letter Report.” 1964, Bates no. 301084074-301084078. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=hjhf0201 (accessed April 11, 2017), 1.
- 44. Battelle Memorial Institute, “Project Ariel. Letter Report,” 2.
- 45. H. Geissbühler, “Project Ariel (Biological Part).” 1964, Bates no. 100175581–100175583. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=ssbl0199 (accessed April 11, 2017)
- 46. J. F. Pankow, W. Luo, A. D. Tavakoli, C. Chen, and L. M. Isabelle, “Delivery Levels and Behavior of 1, 3-Butadiene, Acrylonitrile, Benzene, and Other Toxic Volatile Organic Compounds in Mainstream Tobacco Smoke From Two Brands of Commercial Cigarettes,” Chem Res Toxicol 17, no. 6 (2004): 805–813; J. F. Pankow, “A Consideration of the Role of Gas/Particle Partitioning in the Deposition of Nicotine and Other Tobacco Smoke Compounds in the Respiratory Tract,” Chem Res Toxicol 14, no. 11(2001): 1465–1481; B. Caldwell, W. Sumner, and J. Crane, “A Systematic Review of Nicotine by Inhalation: Is There a Role for the Inhaled Route?” Nicotine Tob Res 14, no. 10 (2012): 1127–1139. [DOI] [PubMed]
- 47. R. Funk and H. Schachner, “Project Ariel. Letter-Report.” 1964, Bates no. 100175550–100175554. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=jsbl0199 (accessed April 11, 2017). Hans Geissbühler, “Final Report on Biological Part of Project Ariel.” 1964, Bates no 100335776-100335807. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=psdn0208 (accessed April 11, 2017), Table 3.
- 48. D. G. Felton, “Annual Report 1964/65.” 1965, Bates no. 105429788-105429870. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=qgvw0208 (accessed April 11, 2017), 1.
- 49. I. Hughes, P. J. Nichol, N. E. Willis, and R. G. Hook, “Nicotine Administration: Ariel Smoking Devices: Report No Rd 410-R.” 1966, Bates no. 401059276–401059286. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=tgvx0208 (accessed April 11, 2017)
- 50. Ibid., 3.
- 51. Ibid., 5.
- 52. Ibid., 4.
- 53. Charles Ellis and David Williamson, Smoking Device; Smoking Devices. US Patent 3 356 094, filed December 5, 1967.
- 54. A. Yeaman, “Re: Ariel.” 1970, Bates no. 1118–1201. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=njgh0097 (accessed April 11, 2017); H. Rennie, “Note of Telephone Call at About 12 - 30 PM on, Tuesday, 12th July 1966.” 1966, Bates no 301121143. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=xrmx0199 (accessed April 11, 2017)
- 55. S. Green, “Ariel.” 1966, Bates no. 110315695. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=xngy0205 (accessed April 11, 2017); I. Hughes, “[Memo from IW Hughes to SJ Green Regarding Samples of Ariel Cigarettes].” 1966, Bates no. 107623978. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=xmcn0197 (accessed April 11, 2017)
- 56. R. R. Johnson, “Current Chemistry Research at Southampton.” 1967, Bates no. 500012128-500012142. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=yhbw0136 (accessed April 11, 2017), 10.
- 57. S. Green, “Research Conference Held at Kronberg, 2nd – 6th June, 1969.” 1969, Bates no. 1169–1201. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=yqgh0097 (accessed April 11, 2017), 9.
- 58. Hutchison et al., “Charles Drummond Ellis,” 225f.
- 59. S. Green, “Ariel,” 1970, Bates no. 107623970. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=nyjd0040 (accessed April 11, 2017)
- 60. A. Yeaman, “Implications of Battelle Hippo I & II and the Griffith Filter.” 1963, Bates no. 1802–1805. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=hrwh0097 (accessed April 11, 2017)
- 61. Royal College of Physicians of London, Smoking and Health. Summary and Report of the Royal College of Physicians of London on Smoking in Relation to Cancer of the Lung and Other Diseases (New York, NY: Pitman Publishing, 1962); Surgeon General’s Advisory Committee on Smoking and Health, Smoking and Health. Report of the Advisory Committee to the Surgeon General of the Public Health Service (Washington, DC: US Department of Health, Education, and Welfare, 1964); Brandt, Cigarette Century, ch. 8.
- 62. Hutchison et al., Charles Drummond Ellis, 1980.
- 63. A. L. Heard, “Notes on RJR Smokeless Cigarette.” 1987, Bates no. 401032449–401032450. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=qsfd0199 (accessed April 11, 2017)
- 64. Slade, “Nicotine Delivery Devices,” 18.
- 65. Brandt, Cigarette Century, 369–375.
- 66. J. Slade, L. A. Bero, P. Hanauer, D.E. Barnes, and S. A. Glantz, “Nicotine and Addiction. The Brown and Williamson Documents,” JAMA 274, no. 3 (1995): 225–233; A. Stanton and S. Glantz, The Cigarette Papers (Berkeley: University of California Press, 1996), 74–77. [PubMed]
- 67. C. Ellis, “The Smoking and Health Problem.” 1962, Bates no. 100427861-100427883. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=hkbj0195 (accessed April 11, 2017), 2.
- 68. Proctor, Golden Holocaust, chs. 15–18; Brandt, Cigarette Century, chs. 6, 7.
- 69. Proctor, Golden Holocaust, chs. 19 and 22.
- 70. James Graham entertained such an idea after hearing about aerosol smoking devices at a conference. James Graham, “Nicotine and Smoking,” BMJ 4, no. 5729 (1970): 244.
- 71. The most important change to reduce irritation seems to be that e-cigarettes use propylene glycol and sometimes glycerol as carrier compounds. Grana et al., “E-Cigarettes a Scientific Review.”.