FIGURE 3.
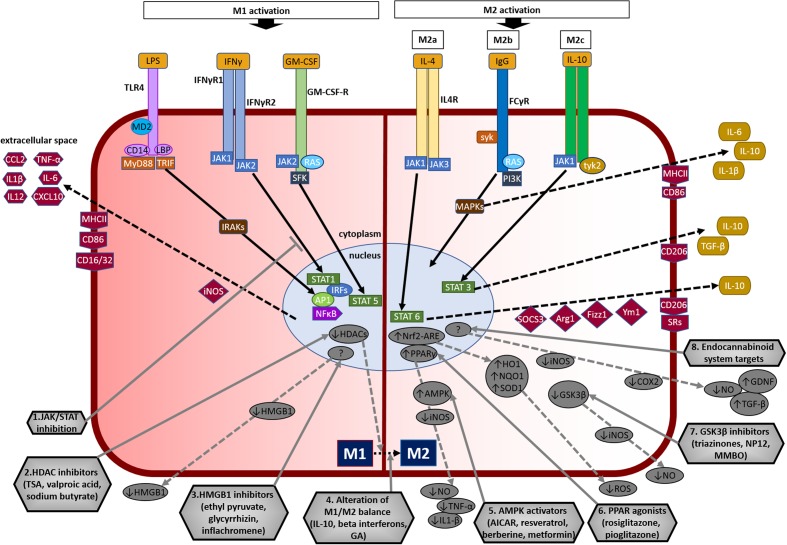
Overview of microglial M1 and M2 signaling in neurodegeneration, and potential targets for neuroprotection. The left side of the figure compartment shows M1 microglial phenotype and its major signaling pathways. LPS binds to TLR4 on the cell surface which is coupled to MD2 (TLR/MD2) with participation of co-receptors CD14 and LBP (LPS-binding protein), activates interleukin-1 receptor-associated kinases (IRAKs) through MyD88 and TRIF that causes translocation of transcription factors such as NF-κB, STAT5, activator protein-1 (AP1), and interferon regulatory factors (IRFs) to the nucleus. M1 activation by IFN-γ occurs through IFN-γ receptors 1 and 2 (IFN-γR1/2) leading to the recruitment of Janus kinase 1 and 2 (JAK1/2) that phosphorylate and translocate STAT1 and IRFs to the nucleus. M1 activation stimulation through granulocyte-macrophage colony-stimulating factor (GM-CSF) occurs when GM-CSF binds to its receptor GM-CSF-R, which activates rat sarcoma oncoproteins (RAS), JAK2, and SFK, and causes translocation of STAT5 to the nucleus. The translocation of NF-κB, STAT1, STAT5, AP1, and IRFs to the nucleus causes upregulation of intracellular iNOS and cell surface markers (CD86, CD16/32, MHC-II). M1 stimulation also causes transcriptional upregulation of M1-associated pro-inflammatory cytokines (IL-1β, IL-6, IL-12, TNF-α) and chemokines (CCL2, CXCL10). The right side of the figure compartment shows various M2 microglial phenotypes and major signaling pathways involved. M2 activation can be classified into M2a, M2b, and M2c. M2a state is induced mainly by IL-4. IL-4 binds to IL-4R, which stimulates JAK1 or JAK3 that causes translocation of STAT6 to the nucleus leading to transcription of M2a-associated genes including IL-10, cell surface markers (CD206, scavenger receptors, SRs), and intracellular components such as suppressor of cytokine signaling 3 (SOCS3), Ym1 (chitinase-like protein) and Fizz1 (found in inflammatory zone). The M2b activation state, which has some M1 response characteristics, is stimulated when TLRs fuse Fcγ receptors, which then bind to IgG (B cells) to derive the M2b phenotype. M2b activation results in secretion of IL-10 and, cell surface markers (CD86, MHC-II). M2c activation is induced by IL-10 which stimulates the IL-10 Receptor 1 and 2 subunits that activates JAK1 leading to the translocation of STAT3 to the nucleus. STAT3 translocation inhibits M1-associated pro-inflammatory cytokines and upregulation of IL-10, TGF-β and the cell surface marker CD206. M2c state plays an important role in immunoregulation, matrix deposition and tissue remodeling. The bottom half of the figure shows potential therapeutic microglial targets for neuroprotection. (1) JAK/STAT inhibition: M1 phenotype is induced via the JAK/STAT signaling pathway and inhibition of this pathway may suppress the downstream M1-associated pro-inflammatory genes. (2) Histone deacetylase (HDAC) inhibitors: Histone acetylation is increased in M1 state that may lead to the expression and release of pro-inflammatory cytokines. HDAC inhibitors prevent neurodegeneration by shifting microglia toward protective M2 phenotype and anti-inflammatory mechanisms. (3) Microglia-produced high-mobility group box-1 (HMGB1) inhibitors: HMGB1 is a pro-inflammatory cytokine released by microglia which is toxic to neurons. HMGB1 inhibitors show neuroprotection by binding to HMGB1 and inhibiting its cytokine-like activity. (4) Alteration of M1/M2 balance: These agents promote the shift M1 pro-inflammatory phenotype toward protective M2 phenotype, and also exhibit neuroprotection by counteracting excessive pro-inflammatory M1 cytokines. (5) Adenosine monophosphate-activated protein kinase (AMPK) activators: AMPK activators act by inhibiting the expression of pro-inflammatory cytokines and iNOS by reducing NF-κB activation. (6) Peroxisome proliferator-activated receptor (PPAR) agonists: PPAR agonists exhibit neuroprotection by upregulating AMPK and protective genes, reducing oxidative damage, maintaining mitochondrial function and anti-inflammatory mechanisms. (7) Glycogen synthase kinase-3 β (GSK3β) inhibitors: GSK3β regulate microglial migration, inflammation, and neurotoxicity through astrocytes. GSK3β inhibitors decrease inflammation by reducing iNOS expression and release of nitric oxide (NO). (8) Endocannabinoid system targets: Agents that target the endogenous cannabinoid ligands anandamide and 2-arachidonoylglycerol (2-AG) increase TGF-β, arginase 1 and glial cell line-derived neurotrophic factor (GDNF), and reduce iNOS and COX-2, expression. AICAR, 5-amino-4-imidazole carboxamide riboside; GA, glatiramer acetate; HO-1, heme oxygenase 1; MMBO, 2-methyl-5-methylsulfinylphenyl-1-benzofuranyl-1,3,4-oxadiazole; NP-12, tideglusib; NQO1, NAD(P)H quinone dehydrogenase 1; Nrf2, nuclear erythroid 2-related factor 2; ARE, antioxidant response element; SOD1, superoxide dismutase 1; TSA, trichostatin A.