Abstract
Background Parasellar invasion of pituitary adenomas (PAs) into the cavernous sinus (CS) is common. The management of the CS component of PA remains controversial.
Objective The objective of this study was to analyze CS involvement in PA treated with endoscopic endonasal approaches, including incidence, surgical risks, surgical strategies, long-term outcomes, and our treatment algorithm.
Methods We reviewed a series of 176 surgically treated PA with particular attention to CS involvement and whether the CS tumor was approached medial or lateral to the internal carotid artery.
Results The median duration of follow-up was 36 months. Macroadenomas and nonfunctional adenomas represented 77 and 60% of cases, respectively. CS invasion was documented in 23% of cases. CS involvement was associated with a significantly diminished odds of gross total resection (47 vs. 86%, odds ratio [OR]: 5.2) and increased the need for subsequent intervention (4 vs. 40%, OR: 14.4). Hormonal remission was achieved in 15% of hormonally active tumors. Rates of surgical complication were similar regardless of CS involvement.
Conclusion Our tailored strategy beginning with a medial approach and adding lateral exposure as needed resulted in good outcomes with low morbidity in nonfunctional adenomas. Functional adenomas involving the CS were associated with low rates of hormonal remission necessitating higher rates of additional treatment.
Keywords: endoscopic, cavernous sinus, pituitary adenomas
Introduction
Pituitary adenomas (PAs) frequently grow beyond the sella; this tumor extension can be parasellar, infrasellar, and/or suprasellar. PA parasellar extension into the cavernous sinus (CS) occurs in 10 to 40% of surgical cases. 1 2 3 4 5 The management of the CS component of PA is controversial. Surgical resection of tumor in the CS risks complications, 6 7 but these can be minimized by thorough understanding of the microsurgical anatomy of the CS and endoscopic techniques. 8 9 10 11 Here, we report our experience treating PAs extending into the CS, analyze the incidence of CS extension, surgical risks, and long-term results of endoscopic treatment, and propose a treatment algorithm.
Methods
We retrospectively reviewed the Stanford University Pituitary Database for surgical cases of PA between 2007 and 2012. This study was approved by our institutional review board committee (IRB-3054; 6208). We identified 183 patients who underwent endoscopic resection of pituitary by the senior authors (P.H. and G.R.H.). Seven charts were excluded for missing data resulting in n = 176 for further analysis. All had pathological confirmation of the diagnosis of PA. Other lesions were excluded from this analysis.
Preoperative coronal T1 magnetic resonance imaging (MRI) with contrast was used to evaluate the Knosp grading system. CS involvement by tumor was defined as tumor extension beyond the medial wall of the CS. Specific criteria were Knosp grade 1 or 2 tumor extension on preoperative coronal T1 MRI with contrast which was confirmed by intraoperative evaluation, Knosp grade 3 or 4 tumor extension on preoperative coronal T1 MRI with contrast, asymmetrical expansion of the CS by tumor on preoperative coronal T1 MRI with contrast, or intraoperative observation of CS invasion regardless of preoperative radiological evaluation. Tumors were also analyzed in regard to size (macroadenoma > 1 cm), hormonal secretion, and immunohistochemical staining.
Our sinonasal approach involves removal of the superior turbinate in all cases and the middle turbinate when more lateral exposure is needed. The pterygopalatine fossa (PPF) is exposed from medial to lateral as needed ( Fig. 1 ). 12 Observation and Doppler ultrasonography are utilized to localize the internal carotid artery (ICA). A nasoseptal flap, harvested at the end of the procedure, is used for reconstruction when a substantial cerebrospinal fluid (CSF) fistula has been created. A lumbar drain is used perioperatively for macroadenomas with significant suprasellar extension not capped by gland or diaphragma sella.
Fig. 1.
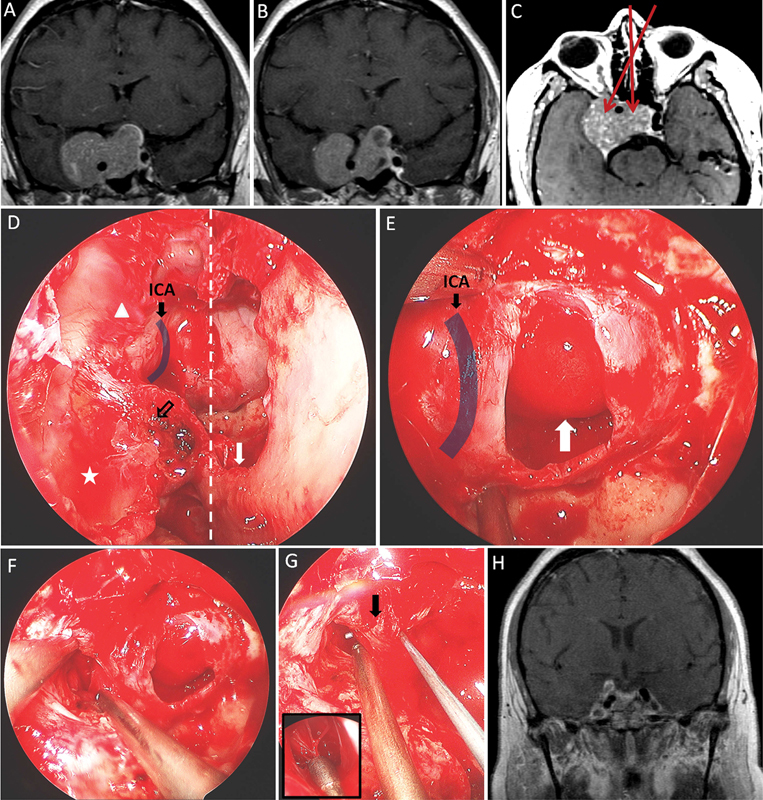
Illustrative case 1. A 35-year-old woman presenting with headache and diplopia was found to have superior visual field deficits in her right eye in addition to right partial CNs III and VI palsies. Examination and laboratory evaluations revealed no evidence of hormonal hypersecretion. Following surgical intervention as illustrated ( A–H ), the patient's symptoms fully resolved. ( A, B ) Coronal T1 MRIs with gadolinium injection showed a sellar lesion with parasellar and suprasellar extension. ( C ) Axial T1 MRI with gadolinium injection demonstrated the same lesion; the trajectories of the approaches utilized are highlighted (red arrows). The tumor was resected through an endoscopic endonasal route and a midline approach, medial to the ICA, and a lateral approach, lateral to the ICA. Pathology showed nonstaining pituitary adenoma with atypical features. ( D ) Intraoperative endoscopic view documenting sinonasal exposure (midline, dotted line) with a wide septostomy (white arrow) performed to allow binostril instrumentation and visualization. The maxillary sinus (star) and the posterior ethmoidal sinus (triangle) were opened to sufficiently expose the lateral extension of the sphenoid sinus. The PPF (empty black arrow) was partially opened. ( E ) Tumor resection began medial to the ICA; previously suprasellar arachnoid dome invaginates the sella evacuated of tumor (white arrow). ( F ) The parasellar (cavernous) component was resected through a dural opening lateral to the ICA. ( G ) A nerve hook could be passed posterior to the ICA (arrow). At the end of the resection, the sellar space and the cavernous space were connected. Septations usually associated with tumor penetration of the medial CS wall are demonstrated (enlarged). ( H ) Postoperative coronal T1 MRIs with gadolinium injection demonstrated the significant extent of surgical resection. CN, cranial nerve; ICA, internal carotid artery; MRI, magnetic resonance imaging; PPF, pterygopalatine fossa.
Tumor in the CS was approached medial to ICA (medial approach), lateral to the ICA through the anterior wall of the CS (lateral approach), or in both directions. In general, we first resected sellar and suprasellar tumor and then worked laterally, following tumor through the medial wall of the CS to resect the CS portion. The bone covering the anterior face of the CS and the cavernous ICA was removed to permit lateral deflection of the anterior CS so as to gain a more direct medial-to-lateral view of the medial wall and any tumor-related defect in it, the region behind the ICA siphon, and the more lateral parts of the CS. Angled endoscopic lenses often improve visualization of lateral structures.
When substantial tumor was inaccessible through this medial-to-lateral approach, a direct lateral approach was added. A lateral approach was most commonly used to access tumor lateral to the ICA, particularly for an hormonally active tumor that had gained this location without opening a wide route of access across the medial wall of the CS ( Figs. 1 and 2 ).
Fig. 2.
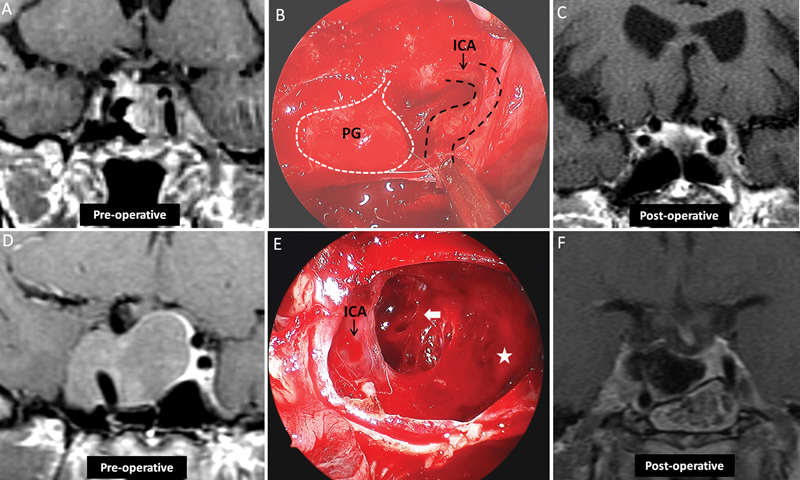
Illustrative cases 2 and 3. ( A–C ) A 75-year-old woman presented with epistaxis. Neurologic exam revealed no deficits, and no evidence of hormonal hypersecretion was demonstrated upon clinical or laboratory evaluation. ( A ) Coronal T1 MRIs with gadolinium injection demonstrates a sellar lesion with parasellar extension. ( B ) Endoscopic view revealed the surgical cavity following use of a midline approach for tumor removal, with the PG pushed to the right and the ICA partially exposed. ( C ) Coronal T1 MRI sequences with gadolinium injection taken 1 year postoperatively showed the extent of resection. The patient required no further treatment. ( D–F ) A 32-year-old woman with acromegaly failed medical treatment. ( D ) Coronal T1 MRI with gadolinium demonstrated a sellar lesion with rightward parasellar extension. Intraoperative endoscopy, using a 45-degree endoscope, demonstrates the resection cavity after tumor removal utilizing a midline approach. The sellar space (star) is connected to the (arrow) through the medial wall of the CS behind the ICA. ( F ) Three-month follow-up coronal T1 MRIs demonstrates the extent of resection, with tumor residual documented lateral to the ICA. The patient's acromegaly improved but subsequently required further treatment. CS, cavernous sinus; ICA, internal carotid artery; MRI, magnetic resonance imaging; PG, pituitary gland.
Even in cases in which tumor appeared to be exclusively intracavernous, anterior sellar dura was opened so that the medial wall could be inspected and any small fragment of glandular tumor and involved medial wall could be resected. If the medial wall was not involved, the anterior CS dura was incised (by a vertical incision parallel to the inferior-to-superior course of the ICA) medial to, lateral to, or on both sides of the CS ICA as permitted by tumor displacement of the ICA from the line of planned incision.
Patients were seen 10 days after surgery, and postoperative imaging was performed 3 months after surgery. Long-term follow-up consisted of an annual outpatient clinical follow-up and MRI. In addition, patients were evaluated in rhinology clinic 10 days and 3 months, postoperatively, and then as needed. Neuroendocrinology follow-up occurred every 3 months in the first year and yearly thereafter.
All data analysis documented here were performed using IBM SPSS (Version 22.0, IBM). Statistical tests included Pearson's chi-square tests of association between predictor and outcome variables, followed by univariate and logistic regression analyses, with a p -value ≤ 0.05 considered significant in all instances. Variables tested as potential predictive factors included age older than 50 years, gender, previous surgery, presence of macroadenoma, degree of surgical resection, and CSF leak.
Results
The median patient age at the time of the first surgical intervention was 50 years (range, 18–89 years). Median duration of follow-up was 36 months (range, 1–78 months). The most common presenting symptoms included hormonal imbalances in 37% and headaches in 26% of the cases. Macroadenomas represented 77% of the full series; 60% of the adenomas were hormonally inactive; and 23 tumors were recurrent after previous surgery ( Table 1 ).
Table 1. Patient characteristics.
| Total number of patients | 176 |
| The median age (range) | 50 y (18–89) |
| Male:female | 1:1.1 |
| N (%) | |
| Presenting symptoms | |
| Hormonal disturbance | 66 (38) |
| Headache | 48 (27) |
| Vision impairment | 37 (21) |
| Incidental | 28 (16) |
| Apoplexy | 12 (7) |
| Other | 8 (5) |
| Size | |
| Microadenoma (< 1 cm) | 41 (23) |
| Macroadenoma (< 1 cm) | 135 (77) |
| Tumor type | |
| Non-functional | 106 (60) |
| Prolactin secreting | 27 (15) |
| GH secreting | 24 (14) |
| ACTH secreting | 17 (10) |
| TSH secreting | 2 (1) |
| Previous surgery | 23 (13) |
Abbreviations: ACTH, adrenocorticotropic hormone; GH, growth hormone; TSH, thyroid-stimulating hormone.
The incidence of CS involvement was 23%. Patients were stratified into two groups based on the presence (Group A, n = 40) or absence (Group B, n = 136) of CS involvement. These two groups did not differ significantly with respect to age or gender ( Table 2 ). Hormonal disturbances and headaches were the most common presenting symptoms for both groups. Thirteen percent of Group A and 4% of Group B patients presented with diplopia (odds ratio [OR]: 3.7, 95% confidence interval [CI]: 1.0–13.7, p = 0.03). Ninety-five percent of Group A tumors were macroadenomas compared with 71% of group B tumors (OR: 7.6, 95% CI: 1.8–33.0, p = 0.002). Nonfunctional adenomas comprised 67 and 58% of tumors in Groups A and B, respectively, a difference that did not reach statistical significance ( Supplementary Fig. 1 ). Nonfunctional adenomas (25%), growth hormone (GH)–secreting adenomas (29%), and adrenocorticotropic hormone (ACTH)–staining adenomas (40%) had relatively high rates of CS invasion, but this reached statistical significance only for ACTH-staining adenomas as documented in Table 2 (OR: 2.6, 95% CI: 1.0–6.9, p = 0.05).
Table 2. Risk factors associated with cavernous sinus invasion.
| Total | Rate of cavernous sinus involvement | ||||
|---|---|---|---|---|---|
| N | No n (%) |
Yes n (%) |
OR (95% CI) | p -Value | |
| Total | 176 | 136 | 40 | – | – |
| Age < 50 y | 86 | 71 (52) | 15 (37.5) | 1.8 (0.9–3.8) | 0.1 |
| Gender: male | 83 | 62 (46) | 21 (52.5) | 1.3 (0.6–2.7) | 0.4 |
| Presenting symptoms | |||||
| Apoplexy | 12 | 10 (7) | 2 (5) | 0.7 (0.1–3.2) | 0.6 |
| Hormonal disturbance a | 66 | 56 (41) | 10 (25) | 0.5 (0.2–1.0) | 0.06 |
| Vision loss | 27 | 19 (14) | 8 (20) | 1.5 (0.6–3.8) | 0.3 |
| Double vision | 10 | 5 (4) | 5 (13) | 3.7 (1.0 – 13.7) | 0.03 |
| Headache | 48 | 38 (28) | 10 (25) | 0.9 (0.4–1.9) | 0.7 |
| Incidental | 28 | 21 (15) | 7 (18) | 1.2 (0.5–3.0) | 0.7 |
| Size | |||||
| Macroadenoma (> 1 cm) | 135 | 97 | 38 | 7.6 (1.8 – 33) | 0.002 |
| Tumor type | |||||
| Nonfunctional | 106 | 79 | 27 | 1.5 (0.7–3.2) | 0.3 |
| Prolactin secreting | 27 | 24 | 3 | 0.4 (0.1–1.3) | 0.1 |
| GH secreting | 24 | 17 | 7 | 1.5 (0.6–3.9) | 0.4 |
| ACTH secreting | 17 | 14 | 3 | 0.7 (0.2–2.6) | 0.6 |
| TSH secreting | 2 | 2 | 0 | – | – |
| Tumor staining by IHC | |||||
| ACTH staining | 20 | 12 | 8 | 2.6 (1.0–6.9) | 0.05 |
Abbreviations: ACTH, adrenocorticotropic hormone; CI, confidence interval; GH, growth hormone; IHC, immunohistochemical; OR, odds ratio; TSH, thyroid-stimulating hormone.
Including hypersecretion, stalk effect, and hypopituitarism.
In preoperative radiological evaluation, 7.5, 15, 37.5, and 27.5% of tumors were Knosp grades 1 to 4, respectively. Five tumors (12.5%) with CS invasion that showed asymmetrical expansion of the CS on MRI were included in Group A (CS invasion group). Three tumors fit our radiological criteria for CS involvement but had no documentation of CS invasion in the operative report, one Knosp grade 3 tumor, one Knosp grade 4 tumor, and one tumor with asymmetrical CS expansion. On the contrary, three Knosp grade 1 PAs showed no signs of CS invasion preoperatively but CS invasion was clearly seen intraoperatively.
CS involvement was associated with a lower rate of gross total resection of PA, as evidenced by a gross total resection rate of 47% in Group A versus 86% of Group B ( Table 3 ; OR: 5.2, 95% CI: 2.4–11.5, p < 0.0001). Group A patients were 10 times more likely to require further treatment for residual tumor at a rate of 40 versus only 4% in cases with no CS involvement (OR: 14.4, 95% CI: 5.1–40.6, p < 0.05). The most common treatment for residual disease was stereotactic radiosurgery ( n = 12). Only 15% (2/13) of hormonally active tumors in Group A had hormonal remission compared with 63% (36/57) in Group B. The discrepancy in endocrinologic remission was greatest for ACTH-secreting adenomas: 0% (0/3) for Group A versus 79% (11/14) for Group B ( Supplementary Table 1 ).
Table 3. Surgical results stratified by cavernous sinus involvement.
| Total | Rate of cavernous sinus involvement | ||||
|---|---|---|---|---|---|
| n (%) | No n (%) |
Yes n (%) |
OR (95% CI) | p -Value | |
| Total number | 176 (100) | 136 (77) | 40 (23) | – | – |
| Resection | |||||
| Gross total | 137 (78) | 117 (86) | 19 (47) | 5.2 (2.4 – 11.5) | < 0.0001 |
| Further intervention required | |||||
| Yes | 22 (12) | 6 (4) | 16 (40) | 14.4 (5.1 – 40.6) | < 0.0001 |
| Further intervention: surgery | |||||
| Yes | 7 (4) | 3 (2) | 4 (10) | 4.9 (1.1 – 23.0) | 0.03 |
| Further intervention: radiation | |||||
| Yes | 15 (9) | 3 (2) | 12 (30) | 19.0 (5.0 – 71.8) | < 0.0001 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Rates of perioperative complications were similar in both groups. CS involvement was not associated with increased morbidity ( Supplementary Table 2 ). Postoperative CSF leakage occurred in 7.5% of Group A and 3.6% in Group B, a difference that did not reach statistical significance. One patient in each group suffered postoperative suprasellar bleeding. No patient in either group developed a new cranial nerve deficit. One patient in Group A sustained injury to the ICA whose bleeding was controlled intraoperatively without causing new permanent neurologic deficit.
Discussion
Parasellar extension of PA into the CS is seen in 10 to 40% of surgical cases. 1 2 3 4 5 High rates of parasellar extension may arise from the single dural layer composition of the medial wall of the CS in direct contrast to the two-layered dura found at all other CS boundaries. 13 Parasellar extension has also been attributed to absence of a true dural layer between sellar contents and the CS 14 or to wall penetration by perforating vessels. 15 16
CS invasion occurred in 23% of patients in our series. This relatively high incidence of CS involvement may reflect our definition of CS involvement or the relatively large size (77% macroadenomas) of the tumors in our series. An important observation is that not all CS invasion was evident on preoperative MRI. Three cases of CS invasion (7.5%) were not recognized on preoperative imaging. On the contrary, in three cases, CS invasion thought present on preoperative MRI was not noted intraoperatively.
In prospective evaluation, parasellar invasion can be missed on preoperative MRI, especially for Knosp grades 1 and 2 tumors. 17 Vieira et al evaluated several factors on preoperative MRI, the presence of normal pituitary tissue between the tumor and the CS, visualization of CS venous components, CS size, bulging of the lateral wall of the CS, displacement of the ICA by tumor, Knosp grade of parasellar extension, and percentage of intracavernous ICA encased by the tumor in attempt to assess invasion of the CS in a prospective fashion. Tumor encasement of the intracavernous ICA on reviewed MRIs was most predictive of finding CS invasion intraoperatively (92.1% sensitivity and 93.5% specificity). 5
Clinical presentations in the two groups were similar except for a higher rate of diplopia in adenomas with CS involvement (4 vs. 13%), a finding consistent with the locations of cranial nerves III, IV, and VI running within the CS and its lateral wall. 13 Increased tumor size, null-cell adenomas, GH-secreting adenomas, ACTH-silent or secretary adenomas, prolactin-secreting adenomas, tumor proliferation, and World Health Organization (WHO) grades 2 and 3 pathologies have previously been associated with CS invasion. 4 9 11 18 19 20 Our series similarly suggests that macroadenomas (diameter > 1 cm) were more likely to extend into the CS (28 vs. 5%). The only significant association between tumor endocrinologic activity and invasion was found for ACTH-staining adenomas. Although GH-secreting adenomas also had a higher rate of invasion, this did not reach statistical significance. In our series, only 3.4% (6/176) of adenomas studied were classified as atypical by WHO 2004 criteria, and only one of these invaded the CS. This low incidence of atypical adenomas is consistent with the incidence of 2.7% found by Saeger et al in a large cohort PAs ( n = 451), but both of these are lower than the quoted 15% incidence found by Zada et al who also noted CS invasion by 83% of their atypical adenomas. 18 21
Management of the CS component of PAs is controversial. Two different strategies are commonly employed. The first, and more aggressive, approach attempts complete surgical removal of the CS portion of the tumor. The relative safety and utility of the endoscopic transnasal approach to the sella and CS have encouraged more aggressive attempts to remove CS tumor, 2 8 9 10 11 22 23 24 despite earlier suggestions of increased rates of morbidity associated with lateral transcranial skull base approaches to the CS. 25 26 27 28 The other, less aggressive, approach is to resect the sellar portion of the tumor and leave the CS portion to observation, reoperation, or radiation if hormonal hypersecretion persists or growth continues. This less aggressive approach is supported by reports of favorable outcomes for PAs treated with radiosurgery. 29 30 31 32 A literature review of radiosurgical treatment of PA found high (68–100%) rates of control of tumor growth and a very wide range (0–100%) of rates of endocrinologic remission. These results were associated with low morbidity with the exception of postirradiation hypopituitarism, whose incidence reached 36% in short-term (less than 5 years) follow-up. 30
Woodworth et al reported their results using a conservative surgical approach that progresses medial to lateral, from within the sella, following tumor laterally into the CS. 10 They eschewed opening dura lateral to the CS ICA because of the increased risk of injury to the ICA and cranial nerves III to VI and the low chance of removing all CS tumor. We begin tumor resection similarly, with a medial-to-lateral approach to CS tumor medial to the ICA ( Fig. 3 ). This is greatly facilitated by the use of an angled endoscope to visualize tumor extending through the medial wall of the CS, particularly that posterior to the ICA. We then add lateral exposure by opening the anterior CS dura lateral to the ICA. This lateral approach requires more extensive sinonasal dissection which includes opening of the posterior ethmoidal sinus, removal of the middle turbinate, and tailored exposure of the PPF in addition to certain identification of the medial-to-lateral location of the ICA within the CS (by preoperative MRI, intraoperative navigation, inspection, and Doppler ultrasonography) before the anterior CS wall is incised.
Fig. 3.
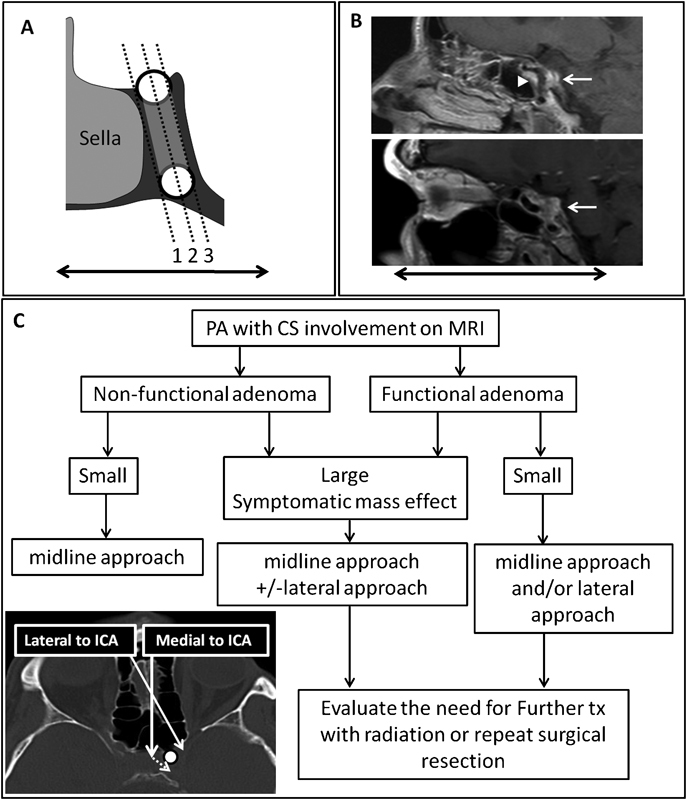
Knosp classification and suggested treatment algorithm. ( A ) The Knosp classifications of PA parasellar extension utilizes medial-to-lateral sequential parasagittal planes to grade CS involvement. ( B ) Oblique parasagittal MRIs demonstrate CS extension in the anterior–posterior plane. The upper MRI shows both anterior medial (triangle) and posterior medial (arrow) compartments. The lower MRI shows the lateral compartment of the CS (arrow). ( C ) Proposed treatment algorithm outlining surgical strategy for PA involving the CS. The main factors influencing surgical approach are tumor size, tumor functional type, and symptomatic mass effect. Tumor involving the CS can be resected either by a midline approach (medial to the ICA) through the medial wall of the CS behind the ICA or via a lateral approach (lateral to the ICA) through the anterior wall of the CS. CS, cavernous sinus; ICA, internal carotid artery; MRI, magnetic resonance imaging; PA, pituitary adenoma; PG, pituitary gland.
The low rate of hormonal remission after surgery and the need for further intervention indicate poorer outcome in cases with CS invasion ( Supplementary Table 1 ). In our series, 40% of cases with CS invasion required further intervention in long-term follow-up compared with 4% in cases without CS invasion. Most of the CS tumors requiring further treatment were hormonally active adenomas. Endocrinologic remission was seen in 15% of cases with CS invasion compared with 63% of cases with no CS invasion. Of the 13 hormonally active PA invading the CS, 8 required further intervention due to tumor growth or lack of endocrinological improvement. A medial approach was the only approach used in these cases because of the small amount of tumor in the CS. A recent study with a similar number of patients and longer follow-up described the surgical outcomes of 56 patients with nonfunctioning PAs and Knosp grades 3 and 4 CS invasion: 7/39 (17.9%) of patients with residual tumor required further intervention because of tumor growth. 33 Nishioka et al reported that the remission rate in patients with GH-producing adenomas and CS invasion was significantly lower than that of patients without CS invasion. Remission was achieved in 15 of 22 (68.2%) of Knosp grade 3 tumors and no (0/4) grade 4 tumors. 34
Since our lateral approach added no morbidity to that seen in cases without CS involvement ( Supplementary Table 2 ), the incremental benefit of a lateral approach and the more extensive resection of CS tumor that it permits should be further evaluated. Our findings are consistent with those of Woodworth et al suggesting lower rates of tumor control and endocrinologic remission in cases with CS invasion. 10 We suggest reserving the lateral approach for large tumors with symptoms of neural or vascular compression whose size cannot be significantly reduced by the medial approach alone ( Fig. 3 ). Aggressive CS exploration may be less strongly indicated for small extensions of hormonally active tumors if a substantial portion tumor is inaccessible. Hormonally active PAs whose hypersecretion persists after surgery should be treated with medication, radiation, or both.
We observed a limited ability of the current preoperative imaging and classification systems to predict CS invasion. This may reflect the reliance of current radiological classifications of parasellar extension on a single coronal image (often that containing the vertical CS ICAs) and resultant less attention to tumor extension occurring in other coronal planes ( Fig. 3 ). 2 5 17 35 Thus, the Knosp classification may be neither an accurate measure of CS extension nor a meaningful predictor of surgical accessibility. Nishioka et al reported CS invasion in 6.5% of Knosp grade 0 and 18.6% of Knosp grade 1 tumors. On the contrary, 13.6% of Knosp grade 3 tumors showed no signs of CS invasion intraoperatively. 34 Therefore, CS invasion is not always predicted by preoperative MRI alone. Zoli et al, in the largest ( n = 402 patients) series assessing the correlation between the revised Knosp classification system and intraoperative CS inspection, 36 observed that 43% of cases with at least Knosp grade 1 invasion lack CS invasion apparent intraoperatively. 36 This absence of CS invasion occurred mostly with Knosp grades 1 and 2 tumors; Knosp grade 3 tumors were highly variable intraoperatively—some had no detectable CS invasion and some invaded the lateral compartment of the CS; and all Knosp grade IV tumors had CS invasion intraoperatively. 36
Some Knosp grade 1 or 2 tumors may have their greatest CS extension posterior to the ascending cavernous ICA (in the posterior medial compartment, Fig. 3B ), a region difficult to reach utilizing a medial approach. Conversely, portions of Knosp grade 3 or 4 tumors in the lateral compartment of the CS ( Fig. 3B ) can often be reached with a medial approach that follows tumor through a corridor posterior to the CS ICA, especially for tumor located behind the anterior ICA genu. A new, modified Knosp classification was proposed in 2015 by Micko et al. It includes further classification of Knosp grade 3 based on the growth of the tumor into grade 3A (superior CS compartment) and grade 3B (inferior CS compartment). 37 In that report, grade 3B was found to be more invasive intraoperatively and was associated with lower rate of endocrinological remission and gross total resection. 37 Further anatomical and prospective surgical studies are required to establish a better radiological classification of tumors with respect to CS invasion and the ability to be removed by surgery. A practical radiological classification of PA invading the CS should consider the tumor's relation to the ICA in multiple coronal, sagittal, and axial planes.
Conclusion
CS involvement by PA is common. Tumors with CS invasion have a higher incidence of diplopia at presentation. ACTH-staining adenomas are associated with an increased risk of CS involvement. Our tailored approach starting with a medial approach followed by lateral exposure, if needed, resulted in good outcomes with low morbidity in hormonally inactive adenomas. We suggest reserving the lateral approach for large tumors with symptomatic CS extension that cannot be significantly reduced by the medial approach. CS involvement by hormonally active adenomas was associated with lower rates of hormonal remission and higher rates of additional treatment. Further prospective evaluation is needed to validate our treatment algorithm and to improve the classification of CS invasion with respect to relevant three-dimensional surgical anatomy and landmarks.
Supplementary Material
Supplementary Fig. 1, Supplementary Table 1 and Supplementary Table 2
Supplementary Fig. 1, Supplementary Table 1 and Supplementary Table 2
References
- 1.Ahmadi J, North C M, Segall H D, Zee C S, Weiss M H. Cavernous sinus invasion by pituitary adenomas. AJR Am J Roentgenol. 1986;146(02):257–262. doi: 10.2214/ajr.146.2.257. [DOI] [PubMed] [Google Scholar]
- 2.Fahlbusch R, Buchfelder M.Transsphenoidal surgery of parasellar pituitary adenomas Acta Neurochir (Wien) 198892(1-4):93–99. [DOI] [PubMed] [Google Scholar]
- 3.Sol Y L, Lee S K, Choi H S, Lee Y H, Kim J, Kim S H. Evaluation of MRI criteria for cavernous sinus invasion in pituitary macroadenoma. J Neuroimaging. 2014;24(05):498–503. doi: 10.1111/j.1552-6569.2012.00710.x. [DOI] [PubMed] [Google Scholar]
- 4.Trouillas J, Roy P, Sturm N et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013;126(01):123–135. doi: 10.1007/s00401-013-1084-y. [DOI] [PubMed] [Google Scholar]
- 5.Vieira J O, Jr, Cukiert A, Liberman B.Evaluation of magnetic resonance imaging criteria for cavernous sinus invasion in patients with pituitary adenomas: logistic regression analysis and correlation with surgical findings Surg Neurol 20066502130–135., discussion 135 [DOI] [PubMed] [Google Scholar]
- 6.Dolenc V V.Transcranial epidural approach to pituitary tumors extending beyond the sella Neurosurgery 19974103542–550., discussion 551–552 [DOI] [PubMed] [Google Scholar]
- 7.Kitano M, Taneda M, Shimono T, Nakao Y. Extended transsphenoidal approach for surgical management of pituitary adenomas invading the cavernous sinus. J Neurosurg. 2008;108(01):26–36. doi: 10.3171/JNS/2008/108/01/0026. [DOI] [PubMed] [Google Scholar]
- 8.Frank G, Pasquini E. Endoscopic endonasal cavernous sinus surgery, with special reference to pituitary adenomas. Front Horm Res. 2006;34:64–82. doi: 10.1159/000091573. [DOI] [PubMed] [Google Scholar]
- 9.Teo C, Wait S.Cavernous sinus invasion by pituitary adenomas: not an issue Skull Base 201121(S01):A058 [Google Scholar]
- 10.Woodworth G F, Patel K S, Shin B et al. Surgical outcomes using a medial-to-lateral endonasal endoscopic approach to pituitary adenomas invading the cavernous sinus. J Neurosurg. 2014;120(05):1086–1094. doi: 10.3171/2014.1.JNS131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B, Wei Y K, Li G L et al. Extended transsphenoidal approach for pituitary adenomas invading the anterior cranial base, cavernous sinus, and clivus: a single-center experience with 126 consecutive cases. J Neurosurg. 2010;112(01):108–117. doi: 10.3171/2009.3.JNS0929. [DOI] [PubMed] [Google Scholar]
- 12.Ajlan A, Achrol A, Soudry E, Hwang P H, Harsh G. Spontaneous sphenoid wing meningoencephaloceles with lateral sphenoid sinus extension: the endoscopic transpterygoid approach. J Neurol Surg B Skull Base. 2014;75(05):314–323. doi: 10.1055/s-0034-1372465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campero A, Campero A A, Martins C, Yasuda A, Rhoton A L., Jr Surgical anatomy of the dural walls of the cavernous sinus. J Clin Neurosci. 2010;17(06):746–750. doi: 10.1016/j.jocn.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Dietemann J L, Kehrli P, Maillot C et al. Is there a dural wall between the cavernous sinus and the pituitary fossa? Anatomical and MRI findings. Neuroradiology. 1998;40(10):627–630. doi: 10.1007/s002340050653. [DOI] [PubMed] [Google Scholar]
- 15.Laws E R., JrPituitary pseudocapsule J Neurosurg 2006104011–2., 2–3 [DOI] [PubMed] [Google Scholar]
- 16.Oldfield E H, Vortmeyer A O. Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg. 2006;104(01):7–19. doi: 10.3171/jns.2006.104.1.7. [DOI] [PubMed] [Google Scholar]
- 17.Knosp E, Steiner E, Kitz K, Matula C.Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings Neurosurgery 19933304610–617., discussion 617–618 [DOI] [PubMed] [Google Scholar]
- 18.Saeger W, Lüdecke D K, Buchfelder M, Fahlbusch R, Quabbe H J, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007;156(02):203–216. doi: 10.1530/eje.1.02326. [DOI] [PubMed] [Google Scholar]
- 19.Scheithauer B W, Gaffey T A, Lloyd R Vet al. Pathobiology of pituitary adenomas and carcinomas Neurosurgery 20065902341–353., discussion 341–353 [DOI] [PubMed] [Google Scholar]
- 20.Thapar K, Scheithauer B W, Kovacs K, Pernicone P J, Laws E R., Jrp53 expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions Neurosurgery 19963804765–770., discussion 770–771 [PubMed] [Google Scholar]
- 21.Zada G, Woodmansee W W, Ramkissoon S, Amadio J, Nose V, Laws E R., Jr Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J Neurosurg. 2011;114(02):336–344. doi: 10.3171/2010.8.JNS10290. [DOI] [PubMed] [Google Scholar]
- 22.Ceylan S, Koc K, Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg. 2010;112(01):99–107. doi: 10.3171/2009.4.JNS09182. [DOI] [PubMed] [Google Scholar]
- 23.Isolan G R, Krayenbühl N, de Oliveira E, Al-Mefty O. Microsurgical Anatomy of the Cavernous Sinus: Measurements of the Triangles in and around It. Skull Base. 2007;17(06):357–367. doi: 10.1055/s-2007-985194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koutourousiou M, Vaz Guimaraes Filho F, Fernandez-Miranda J C, Wang E W, Snyderman C H, Gardner P A.Endoscopic endonasal surgery for tumors of the cavernous sinus: experience of 234 cases J Neurol Surg B Skull Base 201475(S01):A111 [Google Scholar]
- 25.DeMonte F, Smith H K, al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81(02):245–251. doi: 10.3171/jns.1994.81.2.0245. [DOI] [PubMed] [Google Scholar]
- 26.Spallone A, Vidal R V, Gonzales J G. Transcranial approach to pituitary adenomas invading the cavernous sinus: A modification of the classical technique to be used in a low-technology environment. Surg Neurol Int. 2010;1:25. doi: 10.4103/2152-7806.65054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamiya T, Ono Y, Date I, Kawauchi M, Matsumoto K, Ohmoto T. Extradural temporopolar approach for giant pituitary adenomas invading the cavernous sinus and parasellar regions. No Shinkei Geka. 1998;26(09):803–811. [PubMed] [Google Scholar]
- 28.Yasuda A, Campero A, Martins C, Rhoton A L, Jr, de Oliveira E, Ribas G C.Microsurgical anatomy and approaches to the cavernous sinus Neurosurgery 200556(1, Suppl):4–27., discussion 4–27 [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Paeng S, Pyo S, Jeong Y, Lee S, Jung Y.Gamma Knife surgery for invasive pituitary macroadenoma J Neurosurg 2006105(Suppl):26–30. [DOI] [PubMed] [Google Scholar]
- 30.Laws E R, Sheehan J P, Sheehan J M, Jagnathan J, Jane J A, Jr, Oskouian R.Stereotactic radiosurgery for pituitary adenomas: a review of the literature J Neurooncol 200469(1-3):257–272. [DOI] [PubMed] [Google Scholar]
- 31.Shin M, Kurita H, Sasaki T et al. Stereotactic radiosurgery for pituitary adenoma invading the cavernous sinus. J Neurosurg. 2000;93 03:2–5. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 32.Tinnel B A, Henderson M A, Witt T C et al. Endocrine response after gamma knife-based stereotactic radiosurgery for secretory pituitary adenoma. Stereotact Funct Neurosurg. 2008;86(05):292–296. doi: 10.1159/000151717. [DOI] [PubMed] [Google Scholar]
- 33.Ferreli F, Turri-Zanoni M, Canevari F R et al. Endoscopic endonasal management of non-functioning pituitary adenomas with cavernous sinus invasion: a 10- year experience. Rhinology. 2015;53(04):308–316. doi: 10.4193/Rhino14.309. [DOI] [PubMed] [Google Scholar]
- 34.Nishioka H, Fukuhara N, Horiguchi K, Yamada S. Aggressive transsphenoidal resection of tumors invading the cavernous sinus in patients with acromegaly: predictive factors, strategies, and outcomes. J Neurosurg. 2014;121(03):505–510. doi: 10.3171/2014.3.JNS132214. [DOI] [PubMed] [Google Scholar]
- 35.Mohr G, Hardy J, Comtois R, Beauregard H. Surgical management of giant pituitary adenomas. Can J Neurol Sci. 1990;17(01):62–66. doi: 10.1017/s0317167100030055. [DOI] [PubMed] [Google Scholar]
- 36.Zoli M, Milanese L, Bonfatti R et al. Cavernous sinus invasion by pituitary adenomas: role of endoscopic endonasal surgery. J Neurosurg Sci. 2016;60(04):485–494. [PubMed] [Google Scholar]
- 37.Micko A S, Wöhrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg. 2015;122(04):803–811. doi: 10.3171/2014.12.JNS141083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1, Supplementary Table 1 and Supplementary Table 2
Supplementary Fig. 1, Supplementary Table 1 and Supplementary Table 2


