Abstract
Background: Human epidermal growth factor receptor type 2 (HER2) is abundant in a wide variety of tumors and associated with the poor prognosis. Radiolabeled affibodies are potential candidates for detecting HER2-positive lesions. However, laborious multiple-step synthetic procedure and high abdomen background may hinder the widespread use. Herein, cysteinylated ZHER2:342 modified with a new hydrophilic linker (denoted as MZHER2:342) was designed and labeled using 18FAl-NOTA strategies. The biologic efficacy of the novel tracer and its feasibilities for in vivo monitoring HER2 levels were also investigated in xenograft models with different HER2 expressions.
Method: MZHER2:342 was conjugated with MAL-NOTA under standard reaction conditions. The affibody molecule was then radiolabeled with 18FAl complex. The binding specificity of the tracer, 18FAl-NOTA-MAL-MZHER2:342, with HER2 was primarily characterized via in vitro studies. MicroPET imaging were performed in nude mice bearing tumors (SKOV-3, JIMT-1 and MCF-7) after injection. The HER2 levels of xenografts were determined using Western blotting analysis.
Results: 18FAl-NOTA-MAL-MZHER2:342 can be efficiently produced within 30 min with a non-decaycorrected yield of about 10% and a radiochemical purity of more than 95%. In vitro experiments revealed that the modified affibody retained the specific affinity to HER2. PET imaging showed that SKOV-3 and JIMT-1 xenografts were clearly visualized with excellent contrast and low abdomen backgrounds. On the contrary, the signals of MCF-7 tumor were difficult to visualize. The ROI values ranged from16.54±2.69% ID/g for SKOV-3 to 8.42±1.20 %ID/g for JIMT-1 tumors at 1h postinjection respectively. Poor uptake was observed from MCF-7 tumors with 1.71±0.34% ID/g at the same time point. Besides, a significant linear correlation between % ID/g values and relative HER2 expression levels was also found.
Conclusions: 18FAl-NOTA-MAL-MZHER2:342 is a promising tracer for in vivo detecting HER2 status with the advantages of facile synthesis and favorable pharmacokinetics. It may be useful in differential diagnosis, molecularly targeted therapy and prognosis of the cancers.
Keywords: PET, HER2
Introduction
Human epidermal growth factor receptor-2 (HER2) is a 185-kDa transmembrane phosphoglycoprotein belonging to the EGFR family of tyrosine kinases. 1 It plays a key role in the proliferation, differentiation, and migration of cancer via two main signaling pathways (Ras/MAPK and PI3K). HER2 overexpression in cancers is correlated with tumor aggressiveness and worse survival. Assessment of the receptor status is benefited for early detection of tumor recurrence, patient selection for molecularly targeted therapy and prognosis of the disease. 2, 3, 4
Biopsy is the commonly used procedure to determine HER2 expression pattern by ex vivo, however the sampled parts removal from the body may not properly represent the overall tumor characteristics due to heterogeneity of HER2 expression. 5 Non-invasive molecular imaging technology (e.g. SPECT, PET) provides a reliable strategy for in vivo determining HER2 expression via whole-body detection of abnormalities. Conventional 18F-FDG PET has been widely employed for visualizing the cancer tissues according to increased glucose metabolism, while its ability to distinguish tumors with HER2 positive expression was disputed. 6,7
89Zr labeled anti-HER2 monoclonal antibodies such as 89Zr-trastuzumab and 89Zr-pertuzumab etc have been investigated to monitor HER2 levels in malignant tumors. 8,9 Although it showed good imaging properties, low tumor penetration, slow clearance and high radiation exposure preclude the clinical applications. 10,11 A small size targeting proteins, affibody molecules (~6.5 KDa), are alternative candidates for HER2 imaging with high specificity and rapid elimination. The high-contrast images could be obtained within several hours after injection of the radiolabeled affibodies, whereas it may take few days to clear backgrounds with antibodies. 12
Several derives of a HER2-binding affibody, ZHER2:342, have been developed and evaluated in preclinical and clinical SPECT and PET studies. 13, 14, 15-20 For example, 111In-ABY-025 demonstrated noninvasive localization of metastases in patients with breast cancer. 17Compared with SPECT, PET offers improved sensitivity, resolution and quantification. Various PET radioisotopes (68Ga,18F)labeled affibody molecules have shown favorable performances. 19, 20, 21 Among these probes, the 18F-labeled counterparts would be preferred for clinical use due to commercial availability and good imaging properties. Preclinical studies have shown that N-2-(4-18Ffluorobenzamido) ethyl]maleimide labeled Cys-ZHER2:342 (18F-FBEM-ZHER2:342) is specific for detecting HER2-positive lesions and might be useful for monitoring changes of receptor expression to evaluate the response during therapy. 21, 22, 23
However, high unspecific radioactivity accumulation was found to be kept for at least 1 hour in the liver after administration. 21 It may limit the potential for clinical application since the liver is a common metastatic site of cancer. A novel hydrophilic linker, Gly-Gly-Gly-Arg-Asp-Asn, significantly decreased the abdominal background at early time (nearly 30min) postinjection and efficiently improved the imaging qualities 24,25,26. Thus, we hypothesized that a modified cysteinylated ZHER2:342 affibody with the linker, denoted as MZHER2:342, might possess more favorable in vivo pharmacokinetic performances.
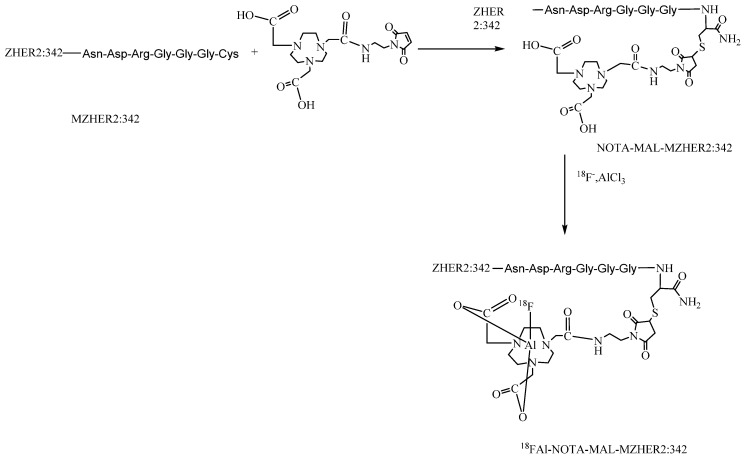
Moreover, the synthesis of 18F-FBEM-ZHER2:342 affibody is tedious for practical use. It requires multiple procedures to achieve the product including HPLC purification for two times. Recently, a simple strategy for preparing 18F-labeled peptides developed by McBride et al via complexation of [18F]aluminum fluoride with 1,4,7-triazacyclononanetriacetic acid (NOTA) -derived peptide has been introduced for tumor imaging. 27, 28, 29 To facilitate the clinical translation, maleimide-NOTA (MAL-NOTA) conjugates of MZHER2:342 was designed and labeled with 18F using the new one-step labeling method (Fig 1). The in vivo biological properties of the 18FAl -labeled MZHER2:342 were investigated in a variety of tumor models and the correlations between quantitative PET data and HER2 status were also preliminarily evaluated.
Figure 1.
Schemes for radiosynthesis of 18FAl-NOTA-MAL-MZHER2:342
Materials and Methods
General
All reagents were analytical grade and were obtained from commercial sources. Cys-ZHER2:342 and Cys-MZHER2:342 were custom made by Apeptide Co., Ltd. (Shanghai, China). MAL-NOTA was purchased from CheMatech (Dijon, France). No-carrier-added [18F]fluoride was obtained from an in-house cyclotron (HM67, Sumitomo Heavy Industries, Ltd.) Analytic and preparative high-performance liquid chromatography (HPLC) were performed according to the previous literatures. 30,31
The peptides was purified on a Waters high-performance liquid chromatography (HPLC) system with a Waters 2998 photodiode array detector (PDA) and a preparative C18 HPLC column (5μm, 250×19 mm, Waters Xbridge). The flow rate is 20ml/min, and the mobile phase changed from 95% solvent A (0.1% trifluoroacetic acid in water) and 5% solvent B (0.1% trifluoroacetic acid in acetonitrile) at 2min to 35% solvent A and 65% solvent B at 21min. The UV absorbance was monitored with the PDA detector at 218 nm.
A Waters Breeze system equipped with a Radiomatic 610TR flow scintillation analyzer (PerkinElmer), a Luna C18 column (5μm, 250×4.6 mm, Phenomenex) and a Waters 2487 dual λ absorbance detector was used for analyze the purities of the compounds. The buffer system were followed: buffer A, 0.1% v/v trifluoroacetic acid in water; buffer B, 0.1% v/v trifluoroacetic acid (TFA) in acetonitrile (ACN); and a gradient of 95% buffer A at 0-2 min to 35% buffer A at 35 min.
Synthesis of NOTA-MAL-MZHER2:342
Cys-MZHER2:342were conjugated with MAL-NOTA under standard reaction conditions as previously described.30,31 Briefly, a solution of 6μmol Cys-MZHER2:342 was mixed with 8μmol MAL-NOTA in 0.2N ammonium acetate solution. After reaction at room temperature for nearly 12hours, the NOTA-conjugated affibody were purified by preparative HPLC. The final product was lyophilized as a white powder. NOTA-MAL-MZHER2:342 was obtained in 50% yield. Matrixassisted laser desorption/ionization (MALDI) time-of-flight (TOF) mass spectrometry (MS) measured m/z 7791.0 for [MH]+ (C337H534N102O107S2, calculated molecular weight, 7790.1).
Radiolabeling
NOTA-MAL-MZHER2:342 was radiolabeled with 18F following procedures 30,31 A solution of NOTA-MAL-MZHER2:342 Affibody molecule (300μg, 38nmol) in sodium acetate buffer (20μL, pH4, 0.2M) was added to a solution of AlCl3 (6μL, 2mM in sodium acetate buffer, pH4, 0.5M) and 18F-fluoride (~3700MBq) in 100μl target water. This mixture was heated for 15min at 100℃. After being diluted with water (10 mL), the reaction solution was transferred to a Varian BOND ELUT C18 column. The cartridge was washed with 10 ml water again, then the desired peptide was eluted with 0.3ml of 10mM HCl in ethanol. The product was reconstituted in saline and passed through a 0.22μmMillipore filter into a sterile vial.
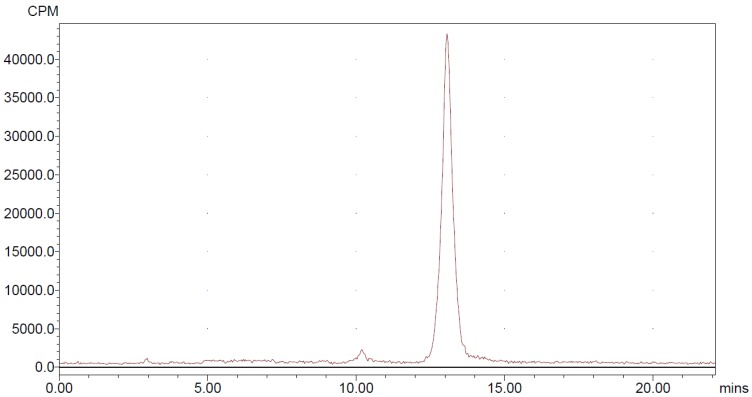
For quality control purposes, a portion of the product was diluted and injected onto an analytical C18 HPLC column to assay for radiochemical purity. The retention time for 18FAl-NOTA-MAL-MZHER2:342 was about 13min.
Cell Lines
Human ovarian cancer (SKOV-3) and breast cancer (JIMT-1, MCF-7) cell lines were purchased from Cell Bank of Shanghai Institutes for Biological Sciences. The cells were cultured in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (GIBCO) (v/v) fetal bovine serum at 37 °C in an atmosphere containing 5% CO2.
Competitive cell-binding assay
The in vitro binding characteristics of the 18FAl labeled affibody molecule were determined using displacement cell-binding assays.21, 30 SKOV-3 tumor cells were cultured into six-well plates ( 2 × 105 cells per well ) until confluency. On the day of the assay, cells were washed with binding buffer (RPMI, 0.5% bovine serum albumin). Subsequently, the cells were incubated with 18F-Al-NOTA-MAL-MZHER2:342 and increasing concentrations of non-labeled Cys-ZHER2:342 Affibody molecules. Removing medium at 2h incubation, cells were washed with binding buffer and collected for determining the radioactivity in a γ-counter (Perkin- Elmer). GraphPad Prism software [version 5.03 for Windows (Microsoft); GraphPad Software] was used to calculate inhibitory concentration of 50% (IC50) values.
Animal Model
All animal experiments were approved by local authorities and were in compliance with the institutional guidelines. Approximately 5×106 tumor cells suspended in 0.2 mL PBS were subcutaneously implanted in the right front flank of 3-4 week-old female Balb/c nude mice (SLAC Laboratory Animal Co., Ltd., China). After tumor size reached 100-300mm3, the animals were used for the following experiments.
MicroPET Imaging
Small-animal PET was performed with a microPET scanner (Siemens Inc.). Under isoflurane anesthesia, the mice were placed prone in the center of the field of view of the scanner and injected into 3.7MBq 18FAl-NOTA-MAL-MZHER2:342 with or without excessive non-labeled Cys-MZHER2:342 via the lateral tail vein. Whole-body scanning was performed at different times after radiotracer injection and a 10-min static PET images were acquired. The quantification analysis of PET images was performed using the same method as previously reported. 30,31
HER2 Western Analysis of Tumor Tissues
After microPET imaging, xenografted tumors were harvested and stored at -70℃. Allowing at least 48h for radioactive decay, the homogenized tissues were lysed using RIPA lysis buffer with 1mM PMSF. The supernatant were collected after centrifugation and used for HER2 Western Analysis. Estimated equal amount of the lysates were loaded in NuPAGE 10% Bis-Tris ReadyGel (Thermo Fisher Scientific Inc.) and transferred onto PVDF membrane (GE Healthcare, Piscataway, NJ, USA), then incubated at room temperature with 5% nonfat milk blocking buffer. The blots were incubated overnight at 4℃ with rabbit anti-HER2 antibody (Beyotime), followed by incubation at room temperature for 1h with horseradish peroxidase-conjugated anti-rabbit secondary antibody (Beyotime). The bands were detected using the BeyoECL plus Western blotting detection system (Beyotime). β-Actin was used as a loading control. Quantification analysis of western gel were performed according the literature.32
Biodistribution Studies
For biodistribution studies, mice were injected with 0.74MBq of each radiotracer via the lateral tail vein. Among them, some mice were coinjected with an excess of unlabeled HER2 affibody (10mg/kg body weight). After euthanized by CO2/O2 asphyxiation, the mice were sacrificed at various time points from 30min to 4h postinjection. Tumor and major organs were dissected and weighed. Activity was measured in a γ-counter. The radioactivity uptakes in the organs were expressed as a percentage of the injected radioactive dose per gram of tissue (% ID/g).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism. Data were analyzed using the unpaired, 2-tailed Student t test. Differences at the 95% confidence level (p< 0.05) were considered to be statistically significant.
Results
Chemistry
Cys-MZHER2:342 was readily conjugated with NOTA-MAL using a previously reported synthesis method. 30,31 Chemical purities of these compounds were >95 % determined by analytical HPLC analysis.
Radiochemistry
NOTA-MAL-MZHER2:342 was easily obtained by site-specific labeling with the Al18F complex in nearly 30min. The non-decay corrected yield was 9.3±1.5% and radiochemical purity of more than 95% (Fig 2).
Figure 2.
HPLC radiochromatogram of purified 18FAl-NOTA-MAL-MZHER2:342
Binding specificity in vitro
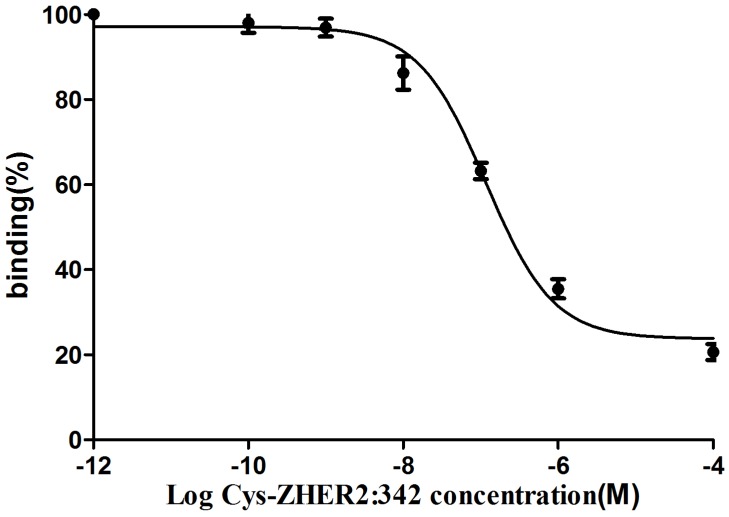
The binding of 18FAl-NOTA-MAL-MZHER2:342 to the HER2 measured on SKOV-3 tumors was inhibited by various concentrations of non-labeled Cys-MZHER2:342, and the IC50 values were 116.71±1.28nM (Fig 3).
Figure 3.
Competitive binding curves of IC50 determination of 18FAl-NOTA-MAL-MZHER2:342 on SKOV-3 cells with Cys-ZHER2:342
Small-Animal PET Imaging
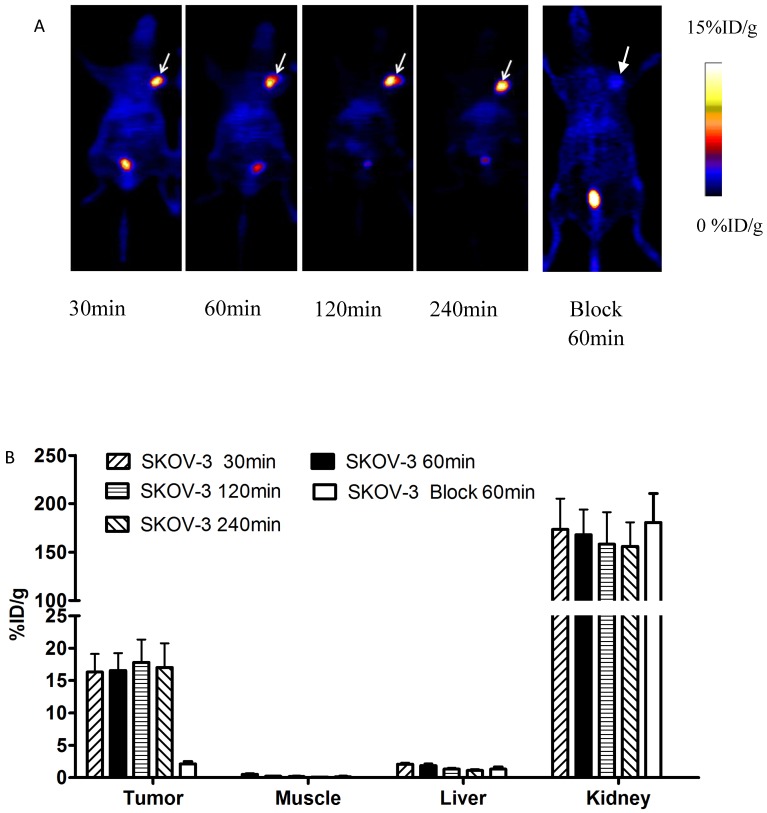
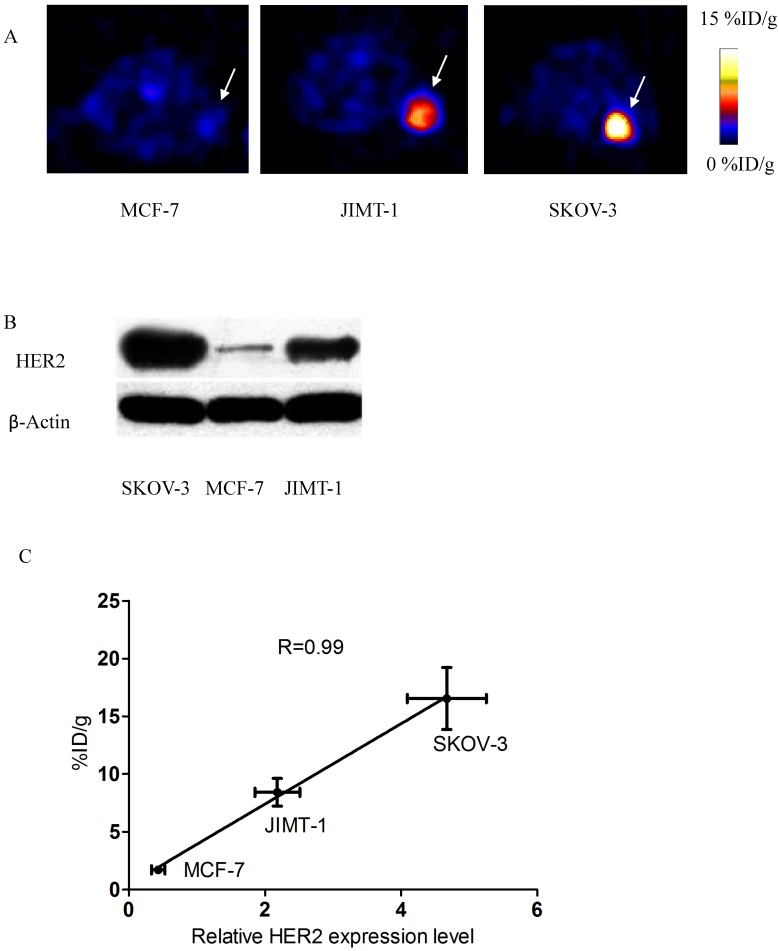
Decay-corrected coronal microPET images of mice bearing SKOV-3 tumors were shown in Fig 4. Axial sections of mice with different levels of HER2 expression are displayed in Fig 5.
Figure 4.
A) Decay-corrected whole-body PET images of mice bearing SKOV-3 xenografts after injection of 18FAl-NOTA-MAL-MZHER2:342 with or without block respectively. B) PET quantification analysis for uptakes of tumor, liver, kidney and muscles for 18FAl-NOTA-MAL-MZHER2:342. ROIs are shown as mean %ID/g ± SD. Tumors are indicated by arrows.
Figure 5.
A) Axial sections 1 h after 18FAl-NOTA-MAL-MZHER2:342 injection in mice bearing tumors. B) Western blots of HER2 in SKOV-3, JIMT-1 and MCF7 xenografted tumors. C) Correlation of PET quantification of tumor uptake at 1 h after injection with relative HER2 expression levels.
SKOV-3 and JIMT-1 xenografts were clearly visualized with good contrast after 1h injection. On the contrary, the tumor signals of MCF-7 tumor was difficult to visualize.
The ROI values ranged from16.54±2.69% ID/g for SKOV-3 to 8.42±1.20%ID/g for JIMT-1 tumors at 1h postinjection respectively. Poor uptake was observed from MCF-7 tumors with 1.71±0.34% ID/g at the same time point. Excessive unlabeled Cys-ZHER2:342 decreased SKOV-3 tumor uptakes to 2.13±0.36% ID/g at 1h postinjection.
Quantitative analysis also revealed that the radioactivity uptake in the kidneys was significantly higher than in normal tissues, which ranged from 167.80±26.15% ID/g to 126.23±25.76% ID/g in mice bearing SKOV-3 and MCF-7 tumors at 1h postinjection respectively.
HER2 Western Analysis of Tumor Tissues
Western blots of HER2 in xenografted tumor samples were shown in Fig 5. HER2 was abundantly expressed in SKOV-3 and JIMT-1 tumors, but was significantly lower in MCF-7 tumors. Quantification analysis of western gel further revealed that relative HER2 expression level was 4.67±0.58, 2.18±0.33 and 0.43±0.10 in SKOV3, JIMT-1 and MCF-7 tumors respectively.
Besides, a significant linear correlation between % ID/g values and relative HER2 expression level obtained from PET quantification and Western analysis respectively was found (R2=0.99, p<0.05) in all tumor-bearing mice.
Biodistribution Studies
The in vivo tumor targeting and imaging profile of 18FAl-NOTA-MAL-MZHER2:342 was further investigated by ex vivo biodistribution studies (Table 1). Biodistribution data were consistent with the imaging data and showed that the uptake of the Al18F labeled MZHER2:342 was 18.32±3.22% ID/g at 30min and kept stable to 17.82±2.46% ID/g in SKOV-3 tumors at 4h postinjection. Accumulation of radioactivities in the JIMT-1 and MCF-7 models was 9.50±2.13%ID/g and 1.96±0.41% ID/g at 1 h after administration. The coinjection of Cys-ZHER2:342 dramatically reduced the SKOV-3 tumor uptake of the tracer to 1.89 ± 0.26% ID/g at 1 h after injection.
Table 1.
Biodistribution of 18FAl-NOTA-MAL-MZHER2:342-Affibody in mice bearing SKOV-3, JIMT-1 and MCF-7 xenografts respectively.
| Organ (%ID/g) |
SKOV-3 | SKOV-3 Block | MCF-7 | JIMT-1 | |||
|---|---|---|---|---|---|---|---|
| 30min | 60min | 120min | 240min | 60min | 60min | 60min | |
| blood | 1.26±0.10 | 0.65±0.08 | 0.39±0.07 | 0.19±0.01 | 0.47±0.06 | 0.55±0.06 | 0.69±0.12 |
| brain | 0.07±0.00 | 0.05±0.00 | 0.03±0.00 | 0.01±0.00 | 0.05±0.00 | 0.04±0.00 | 0.02±0.00 |
| heart | 0.55±0.06 | 0.31±0.06 | 0.19±0.01 | 0.09±0.01 | 0.27±0.03 | 0.36±0.03 | 0.15±0.02 |
| liver | 2.06±0.36 | 1.69±0.23 | 1.19±0.25 | 0.89±0.16 | 1.12±0.19 | 1.35±0.37 | 0.95±0.19 |
| spleen | 0.46±0.09 | 0.36±0.08 | 0.27±0.04 | 0.17±0.01 | 0.61±0.13 | 0.52±0.14 | 0.28±0.05 |
| lung | 1.23±0.24 | 0.67±0.06 | 0.43±0.09 | 0.23±0.02 | 0.81±0.08 | 0.50±0.04 | 0.93±0.09 |
| kidney | 167.16±26.28 | 176.09±12.67 | 183.65±34.52 | 133.65±21.89 | 139.80±19.56 | 149.25±15.52 | 153.88±24.71 |
| stomach | 0.69±0.08 | 0.46±0.04 | 0.47±0.11 | 0.27±0.03 | 0.59±0.07 | 0.39±0.02 | 0.36±0.08 |
| intestine | 0.93±0.23 | 0.56±0.12 | 0.34±0.04 | 0.14±0.02 | 0.79±0.12 | 0.74±0.14 | 0.23±0.09 |
| muscle | 0.47±0.17 | 0.31±0.08 | 0.12±0.03 | 0.08±0.00 | 0.18±0.03 | 0.26±0.06 | 0.22±0.05 |
| pancreas | 0.42±0.08 | 0.24±0.04 | 0.13±0.01 | 0.10±0.01 | 0.21±0.05 | 0.38±0.02 | 0.20±0.01 |
| bone | 1.40±0.17 | 1.34±0.08 | 0.64±0.12 | 0.44±0.05 | 0.99±0.11 | 0.64±0.19 | 1.03±0.15 |
| tumor | 18.32±3.22 | 18.60±3.89 | 19.09±4.77 | 17.82±2.46 | 1.89±0.26 | 1.96±0.41 | 9.50±2.13 |
| Tumor/blood | 14.83±1.73 | 29.80±4.65 | 52.84±11.71 | 94.80±9.35 | 4.15±0.58 | 3.68±0.64 | 14.75±2.65 |
| Tumor/muscle | 47.69±14.10 | 77.75±10.03 | 190.29±34.82 | 240.61±22.95 | 10.04±1.28 | 8.34±2.00 | 47.85±10.56 |
Lower levels of radioactivity were observed in the blood and most other organs. Tumor-to-muscle and tumor-to-blood ratios in mice bearing SKOV-3, JIMT-1 and MCF-7 xenografts were 29.80±4.65, 3.68±0.64, 14.75±2.65 and 190.29±34.82, 8.34±2.00, 47.85±10.56 at 1h p.i. The uptakes in the liver was 1.69±0.23% ID/g at 1h after injection in SKOC-3, and was similar to those in JIMT-1 and MCF-7 models (1.35±0.37% ID/g and 0.95±0.19% ID/g respectively). Among the normal tissues, high activity accumulation was also found in the kidney for all models, which was agreement to the finding from the imaging study. These data confirmed that 18FAl labeled MZHER2:342 were mainly cleared via the urinary system.
Discussion
Molecular imaging with 18F labeled HER2 affibody analogs has shown great potential for tumor characterization and treatment monitoring. Nevertheless, unfavorable hepatic excretion of the tracers may affect the tumor-to-normal tissue ratios and decrease the contrast. Modification the affibody molecule with a hydrophilic linker might effectively alter the pharmacokinetics. 24, 25
In the present study, only one pot reaction was performed in synthesizing the 18FAl labeled derivative of ZHER2:342, and the total preparation time was about 30min with a non-decay-corrected yield at the end of synthesis of 10%. Regarding the aspect of radiosynthesis, the tracer was superior to 18F-FBEM-ZHER2:342 (2 hours preparation time and 6.5% radiochemical yield) 21. Satisfactory radiochemical purities were achieved by analytical radio-HPLC, which revealed that a Sep-Pak C18 cartridge was enough for purification of 18FAl labeled HER2:342 without further HPLC separation.
SKOV-3 tumor is always employed for evaluating the biologic properties of radiolabeled HER2 affibody due to abundant HER2 expression.33,34,35 Thus the HER2 targeting characteristic of 18FAl-NOTA-MAL-MZHER2:342 was initially determined using SKOV-3 models.
In vitro competitive cell-binding experiments showed that the IC50 value of 18FAl-NOTA-MAL-MZHER2:342 was slightly lower than that of 18F-FBEM-ZHER2:342 (116.71 nM vs ~170nM) under similar assay procedure using Cys-ZHER2:342 as competitor with increasing concentrations. 21 It demonstrated that the modification was to have a minimal effect on receptor binding. Also, it indicated that the in vivo tumor-specific uptake characteristics of 18FAl labeled affibody might be more somewhat favorable.
Considering the interesting findings in vitro, the biological characters of the probe were further investigated in living mice bearing tumors using MicroPET imaging. SKOV-3 xenografts are clearly delineated from the surround normal tissues from 30 minutes to 4 hours after injection. Quantification of small-animal PET images showed that nearly 90% of the activity remains in the tumor at 4 h after injection. PET images also showed that even at 4 h after injection, the SKOV-3 tumor uptake of 18FAl-NOTA-MAL-MZHER2:342 can also achieve approximately 15% ID/g, which was significantly higher than those of reported 18F labeled ZHER2:342 affibody.(~6% ID/g at 4h p.i.).21 Meanwhile, coinjection with excess of unlabeled ZHER2:342 affibody dramatically decreased the SKOV-3 tumor uptake and the imaging quality. These results confirmed the excellent receptor targeting specificity of the 18FAl labeled ZHER2:342 affibody as assessed by in vitro methods.
To further determine whether the radiolabeled ZHER2:342 affibody can play a complementary role in assessing target expression, the biological properties of the tracer were also tested in tumors with different HER2 expressions. Similar with the literature, in vivo PET values for each particular tumor model also corresponds well with the HER2 expression levels. 35A significant linear dependence was found between the retained values of the probe and HER2 status in the tumors. The uptake values in SKOV-3 tumors were nearly 2 and 10 folds higher than those of JIMT-1 and MCF-7 tumors respectively, which was consistent with the outcomes of HER2 Western analysis. It means that 18FAl labeled MZHER2:342 affibody molecules may own favorable sensitivity to detect differences in HER2 expression levels.
Biodistribution results were agreement with the PET data analysis, 18FAl-NOTA-MAL-MZHER2:342 rapidly localized in HER2-positive tumors and was eliminated quickly from the blood and normal tissues. The tumor-to-blood and tumor-to-muscle ratios were significantly higher than those of reported 18F labeled HER2 affibody at 60min postinjection (29.80±4.65 and 77.75±10.03 vs 7.5±4.5 and 21±4.7 respectively, p<0.01). At the same time points, 18FAl-NOTA-MAL-MZHER2:342, compared with 18F-FBEM-ZHER2:342, exhibits significantly reduced accumulation in the livers (almost 2% ID/g vs. 5% ID/g at 1 h respectively) 21. It confirmed that modification of the affibody molecules with the hydrophilic linker could reduce hepatobiliary excretion, optimize contrast and improve image quality.
Renal system was the prominent excretion profiles. Enhanced radioactivity accumulation is comparable to those previously reported for 64Cu or 68Ga labeled affibody molecules. 36,37 Considering the physical half-life, positron energy and range, radiation burden of the 18FAl labeled MZHER2:342 affibody may be lower than those of the metal labeled counterparts. Although the kidney is the major dose-limiting organ for the probe, renal protection could be performed by the use of positively charged amino acids, gelofusin, or albumin fragments. Possible effect of the following compounds is under active investigation.
18FAl-NOTA-MAL-MZHER2:342 also produced lower level of radioactivity (<1% ID/g) in the bone than those of 18F-FBEM-ZHER2:342 (2% ID/g) at 1h postinjection as a result of in vivo stability of the tracer. 21Thus, the tracer may decrease the background signal for detection of bone metastasis and radiation dose to the bone marrow.
Conclusion
A novel HER2 affibody, 18FAl-NOTA-MAL-MZHER2:342 has been successfully obtained by a simple route. The tracer shows favorable profiles and excellent tumor imaging quality. It demonstrated that differences in HER2 expression levels can be detected in vivo by PET imaging with the tracer. Therefore, 18FAl labeled ZHER2:342 may be useful in providing specific information on the receptor expression of cancers.
Acknowledgments
This work was partially supported by National Natural Science Foundation (81472749, 81171399, 81101077, 51473071, 81401450, 21401084), CSC Foundation (2011832173), National Significant New Drugs Creation Program (2012ZX09505-001-001), Jiangsu Province Science and Technology Foundation (BE2012622,BE2016632,BK2011166, BE2014609 and BL2012031), Health Ministry of Jiangsu Province Fund (RC2011095,H201529and H201028), Public service platform for Science and technology infrastructure construction project of Jiangsu Province (BM2012066),University of Wisconsin-Madison Department of Medical Physics and Department of Radiology (Radiology R&D Award 1105-002).
References
- 1.Zidan J, Dashkovsky I, Stayerman C. et al. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–6. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–28. doi: 10.1146/annurev-med-042513-015127. [DOI] [PubMed] [Google Scholar]
- 3.Friedlander E, Barok M, Szollosi J. et al. ErbB-directed immunotherapy: antibodies in current practice and promising new agents. Immunol Lett. 2008;116:126–40. doi: 10.1016/j.imlet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Teplinsky E, Muggia F, Targeting HER2 in ovarian, uterine cancers. challenges and future directions. Gynecol Oncol. 2014;135:364–70. doi: 10.1016/j.ygyno.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Tuma RS. Inconsistency of HER2 test raises questions. J Natl Cancer Inst. 2007;99:1064–5. doi: 10.1093/jnci/djm075. [DOI] [PubMed] [Google Scholar]
- 6.Gaykema SB, Schröder CP, Vitfell-Rasmussen J. et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res. 2014;20:3945–54. doi: 10.1158/1078-0432.CCR-14-0491. [DOI] [PubMed] [Google Scholar]
- 7.Marquez BV1, Ikotun OF, Zheleznyak A. et al. Evaluation of (89)Zr-pertuzumab in Breast cancer xenografts. Mol Pharm. 2014;11:3988–95. doi: 10.1021/mp500323d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain M, Kamal N, Batra SK. Engineering antibodies for clinical applications. Trends Biotechnol. 2007;25:307–16. doi: 10.1016/j.tibtech.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Orlova A, Wållberg H, Stone-Elander S. et al. On the selection of a tracer for PET imaging of HER2-expressing tumors: direct comparison of a 124I-labeled affibody molecule and trastuzumab in a murine xenograft model. J Nucl Med. 2009;50:417–25. doi: 10.2967/jnumed.108.057919. [DOI] [PubMed] [Google Scholar]
- 10.Su X, Cheng K, Jeon J. et al. Comparison of two site-specifically (18)F-labeled affibodies for PET imaging of EGFR positive tumors. Mol Pharm. 2014;11:3947–56. doi: 10.1021/mp5003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldwisch J, Tolmachev V. Engineering of affibody molecules for therapy and diagnostics. Methods Mol Biol. 2012;899:103–26. doi: 10.1007/978-1-61779-921-1_7. [DOI] [PubMed] [Google Scholar]
- 12.Löfblom J, Feldwisch J, Tolmachev V. et al. Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010;584:2670–80. doi: 10.1016/j.febslet.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Ahlgren S1, Orlova A, Wållberg H. et al. Targeting of HER2-expressing tumors using 111In-ABY-025, a second-generation affibody molecule with a fundamentally reengineered scaffold. J Nucl Med. 2010;51:1131–8. doi: 10.2967/jnumed.109.073346. [DOI] [PubMed] [Google Scholar]
- 14.Eigenbrot C, Ultsch M, Dubnovitsky A. et al. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc Natl Acad Sci U S A. 2010;107:15039–44. doi: 10.1073/pnas.1005025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altai M, Honarvar H, Wållberg H. et al. Selection of an optimal cysteine-containing peptide-based chelator for labeling of affibody molecules with (188)Re. Eur J Med Chem. 2014;87:519–28. doi: 10.1016/j.ejmech.2014.09.082. [DOI] [PubMed] [Google Scholar]
- 16.Lindberg H, Hofström C, Altai M. et al. Evaluation of a HER2-targeting affibody molecule combining an N-terminal HEHEHE-tag with a GGGC chelator for 99mTc-labelling at the C terminus. Tumour Biol. 2012;33:641–51. doi: 10.1007/s13277-011-0305-z. [DOI] [PubMed] [Google Scholar]
- 17.Sörensen J, Sandberg D, Sandström M. et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J Nucl Med. 2014;55:730–5. doi: 10.2967/jnumed.113.131243. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JM, Zhao XM, Wang SJ. et al. Evaluation of 99mTc-peptide-ZHER2:342 Affibody® molecule for in vivo molecular imaging. Br J Radiol. 2014;87:20130484. doi: 10.1259/bjr.20130484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer-Marek G, Shenoy N, Seidel J. et al. 68Ga-DOTA-affibody molecule for in vivo assessment of HER2/neu expression with PET. Eur J Nucl Med Mol Imaging. 2011;38:1967–76. doi: 10.1007/s00259-011-1810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao Z, Ren G, Liu H. et al. Small-animal PET imaging of human epidermal growth factor receptor positive tumor with a 64Cu labeled affibody protein. Bioconjug Chem. 2010;21:947–54. doi: 10.1021/bc900515p. [DOI] [PubMed] [Google Scholar]
- 21.Kramer-Marek G, Kiesewetter DO, Martiniova L. et al. [18F]FBEM-Z(HER2:342)-Affibody molecule-a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur J Nucl Med Mol Imaging. 2008;35:1008–18. doi: 10.1007/s00259-007-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer-Marek G, Bernardo M, Kiesewetter DO. et al. PET of HER2-positive pulmonary metastases with 18F-ZHER2:342 affibody in a murine model of breast cancer: comparison with 18F-FDG. J Nucl Med. 2012;53:939–46. doi: 10.2967/jnumed.111.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer-Marek G, Gijsen M, Kiesewetter DO. et al. Potential of PET to predict the response to trastuzumab treatment in an ErbB2-positive human xenograft tumor model. J Nucl Med. 2012;53:629–37. doi: 10.2967/jnumed.111.096685. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Gao H, Zhou Y. et al. 18F-Labeled GRPR Agonists and Antagonists: A Comparative Study in Prostate Cancer Imaging. Theranostics. 2011;1:220–9. doi: 10.7150/thno/v01p0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan D, Xu YP, Yang RH. et al. A new (68)Ga-labeled BBN peptide with a hydrophilic linker for GRPR-targeted tumor imaging. Amino Acids. 2014;46:1481–9. doi: 10.1007/s00726-014-1718-y. [DOI] [PubMed] [Google Scholar]
- 26.Pan D, Yan Y, Yang R. et al. PET imaging of prostate tumors with 18F-Al-NOTA-MATBBN. Contrast Media Mol Imaging. 2014;9:342–8. doi: 10.1002/cmmi.1583. [DOI] [PubMed] [Google Scholar]
- 27.McBride WJ, Sharkey RM, Goldenberg DM. Radiofluorination using aluminum-fluoride (Al18F) EJNMMI Res. 2013;3(1):36. doi: 10.1186/2191-219X-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan W, Guo N, Pan D. et al. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54:691–8. doi: 10.2967/jnumed.112.113563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glaser M, Iveson P, Hoppmann S. et al. Three methods for 18F labeling of the HER2-binding affibody molecule Z(HER2:2891) including preclinical assessment. J Nucl Med. 2013;54:1981–8. doi: 10.2967/jnumed.113.122465. [DOI] [PubMed] [Google Scholar]
- 30.Xu Q, Zhu C, Xu Y. et al. Preliminary evaluation of [18F]AlF-NOTA-MAL-Cys39-exendin-4 in insulinoma with PET. J Drug Target. 2015;23:813–20. doi: 10.3109/1061186X.2015.1020808. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Pan D, Zhu C. et al. Pilot study of a novel (18)F-labeled FSHR probe for tumor imaging. Mol Imaging Biol. 2014;16:578–85. doi: 10.1007/s11307-013-0712-1. [DOI] [PubMed] [Google Scholar]
- 32.Miao Z, Ren G, Liu H. et al. PET of EGFR expression with an 18F-labeled affibody molecule. J Nucl Med. 2012;53:1110–8. doi: 10.2967/jnumed.111.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westerlund K, Honarvar H, Norrström E. et al. Increasing the Net Negative Charge by Replacement of DOTA Chelator with DOTAGA Improves the Biodistribution of Radiolabeled Second-Generation Synthetic Affibody Molecules. Mol Pharm. 2016;13:1668–78. doi: 10.1021/acs.molpharmaceut.6b00089. [DOI] [PubMed] [Google Scholar]
- 34.Honarvar H, Westerlund K, Altai M. et al. Feasibility of Affibody Molecule-Based PNA-Mediated Radionuclide Pretargeting of Malignant Tumors. Theranostics. 2016;6:93–103. doi: 10.7150/thno.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer-Marek G, Shenoy N, Seidel J. et al. 68Ga-DOTA-affibody molecule for in vivo assessment of HER2/neu expression with PET. Eur J Nucl Med Mol Imaging. 2011;38:1967–76. doi: 10.1007/s00259-011-1810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altai M, Strand J, Rosik D. et al. Influence of nuclides and chelators on imaging using affibody molecules: comparative evaluation of recombinant affibody molecules site-specifically labeled with ⁶⁸Ga and ¹¹¹In via maleimido derivatives of DOTA and NODAGA. Bioconjug Chem. 2013;24:1102–9. doi: 10.1021/bc300678y. [DOI] [PubMed] [Google Scholar]
- 37.Cheng Z, De Jesus OP, Kramer DJ. et al. 64Cu-labeled affibody molecules for imaging of HER2 expressing tumors. Mol Imaging Biol. 2010;12:316–24. doi: 10.1007/s11307-009-0256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]