Figure 2. Approaches to Novel Target Validation.
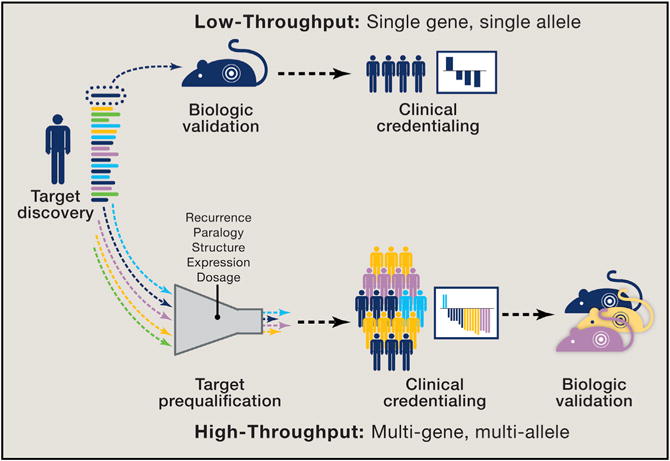
Discovery of a novel target is traditionally followed by biologic validation before proceeding with clinical credentialing. This “monogenic” low-throughput process involves evaluation of one genomic alteration at a time. Next-generation sequencing at the point of care now routinely identifies novel genomic alterations in patients who are in need of new treatment strategies and cannot wait for initial biologic validation. In a “polygenic” high-throughput model, novel genomic alterations observed in patients undergo an initial prequalification using a computational framework that considers allelic recurrence, paralogy, structure, expression, and gene dosage to evaluate the likelihood of clinical relevance. Patients whose tumors harbor qualifying alterations that are identified as potentially activating can then be enrolled in genome-driven clinical trials and the responses observed, potentially providing initial clinical credentialing before biologic characterization. In this model, clinical studies become platforms for exploring the functional consequences of novel genomic alterations detected in the same patient populations.
