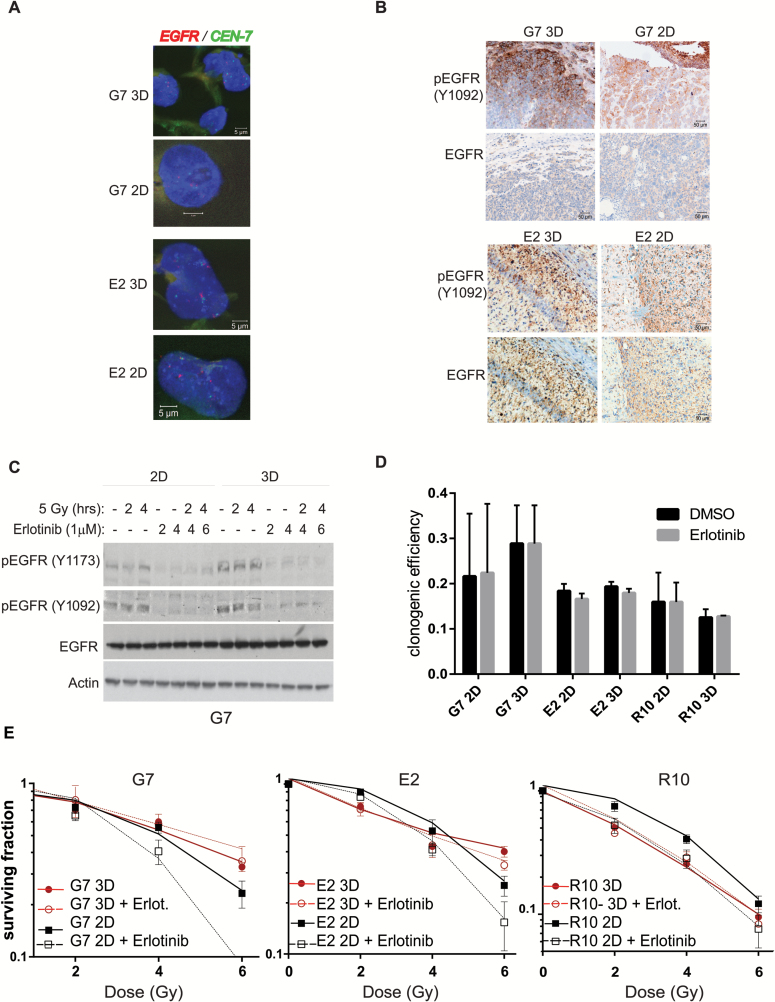
Fig. 4.
Radiosensitization of GSCs by EGFR inhibition is determined by growth conditions. (A) Representative immunofluorescence images of EGFR gene (green) and chromosome 7 centromere (red) staining by FISH assay in G7 and E2 2D and 3D GSC. Nonnuclear staining reflects background autofluorescence. (B) Representative immunohistochemistry images of phosphorylated and total EGFR in G7 and E2 orthotopic tumors from cells grown on 3D or 2D conditions for 7 days. (C) Protein extracts of G7 GSCs grown in 2D or 3D conditions obtained at different time points after treatment with erlotinib (1 µM) and/or ionizing radiation (5 Gy) were analyzed for total and phosphorylated EGFR by western blot. Actin served as loading control. (D) Clonogenic survival efficiency of G7, E2, and R10 cells treated with either vehicle (dimethyl sulfoxide [DMSO]) or erlotinib (1 µM) 20 hours following seeding and left for the duration of the experiment (18 days). Graph depicts mean±SD. (E) Clonogenic survival of G7, E2, and R10 cells grown in 2D and 3D conditions and irradiated with single doses of X-rays (0–6 Gy; n = 3) 2 hours after treatment with erlotinib (1 µM) or DMSO. Erlotinib treatment significantly increased the radiosensitivity of G7, E2, and R10 GSCs under 2D conditions (ANOVA; P < .0001, P = .0006, and P = .0016, respectively). No effect of erlotinib was observed in 3D conditions compared with DMSO (G7 P = .1; E2 P = .1007 and R10 P = .842).