Abstract
Background.
The primary objective of this study was to compare the overall survival (OS) of patients with anaplastic astrocytoma (AA) treated with radiotherapy (RT) and either temozolomide (TMZ) or a nitrosourea (NU). Secondary endpoints were time to tumor progression (TTP), toxicity, and the effect of IDH1 mutation status on clinical outcome.
Methods.
Eligible patients with centrally reviewed, histologically confirmed, newly diagnosed AA were randomized to receive either RT+TMZ (n = 97) or RT+NU (n = 99). The study closed early because the target accrual rate was not met.
Results.
Median follow-up time for patients still alive was 10.1 years (1.9–12.6 y); 66% of the patients died. Median survival time was 3.9 years in the RT/TMZ arm (95% CI, 3.0–7.0) and 3.8 years in the RT/NU arm (95% CI, 2.2–7.0), corresponding to a hazard ratio (HR) of 0.94 (P = .36; 95% CI, 0.67–1.32). The differences in progression-free survival (PFS) and TTP between the 2 arms were not statistically significant. Patients in the RT+NU arm experienced more grade ≥3 toxicity (75.8% vs 47.9%, P < .001), mainly related to myelosuppression. Of the 196 patients, 111 were tested for IDH1-R132H status (60 RT+TMZ and 51 RT+NU). Fifty-four patients were IDH negative and 49 were IDH positive with a better OS in IDH-positive patients (median survival time 7.9 vs 2.8 y; P = .004, HR = 0.50; 95% CI, 0.31–0.81).
Conclusions.
RT+TMZ did not appear to significantly improve OS or TTP for AA compared with RT+ NU. RT+TMZ was better tolerated. IDH1-R132H mutation was associated with longer survival.
Keywords: anaplastic astrocytoma, nitrosourea, radiotherapy, temozolomide
Importance of the Study
This is the first randomized phase III trial of radiotherapy with temozolomide versus nitrosourea in the management of adults with newly diagnosed anaplastic astrocytoma demonstrating no significant difference in survival between the 2 treatment arms. Temozolomide was better tolerated. Mutation in isocitrate dehydrogenase was a major prognostic factor for outcome. This study highlights the importance of incorporating molecular/cytogenetic characterization in clinical trials.
Anaplastic astrocytoma (World Health Organization [WHO] grade III) is a malignant brain tumor that usually progresses to glioblastoma (WHO grade IV). Prior to 1990, the majority of patients enrolled in clinical trials of adjuvant chemotherapy for malignant glioma were diagnosed with glioblastoma, making it difficult to identify a therapeutic effect in other grades and histologies. The large randomized trial of radiotherapy (RT) and adjuvant procarbazine, lomustine, and vincristine (PCV) versus RT alone in high-grade glioma did not show any survival benefit from adjuvant PCV; however, only 17% of the study population had anaplastic astrocytoma.1 Later clinical trials and retrospective reviews stratified outcomes according to tumor grade and histology but produced mixed results on the value of chemotherapy for anaplastic astrocytoma, and its use in this population remained controversial.2,3
NRG Oncology Radiation Therapy Oncology Group (RTOG) 9813 was designed to determine whether the alkylating drug temozolomide (TMZ) was a better adjunct to RT than an alkylating nitrosourea (NU) compound. At the time of study design, TMZ was not an established chemotherapeutic agent, but there were preliminary reports of efficacy in recurrent malignant glioma. In addition, TMZ was convenient to administer and thought to be associated with a more favorable toxicity profile than NU-based chemotherapy.4 TMZ has since shown efficacy in treating recurrent anaplastic astrocytoma5 and in improving overall survival (OS) when combined with RT for glioblastoma,6 but its role in newly diagnosed anaplastic astrocytoma has not been established. Following study initiation, significant interest in assessing whether IDH1 mutation status correlated with outcome in anaplastic astrocytoma developed, and this study afforded an opportunity to evaluate that question as an added secondary endpoint.7 Here we report results of the first study to compare the OS of patients with anaplastic astrocytoma (predominant histology) treated with RT and either TMZ or NU.
Patients and Methods
Eligibility
Patients ≥18 years of age with unifocal, newly diagnosed, centrally reviewed anaplastic astrocytoma or oligoastrocytoma for which the oligodendroglial component was ≤25% were eligible. Other criteria included Karnofsky performance status (KPS) of at least 60 and adequate hematological and laboratory values, and no prior malignancy within 5 years. Patients could not have received prior cranial radiation or chemotherapy or have any preexisting lung disease that would prevent administration or completion of therapy with BCNU (carmustine) or CCNU (lomustine). Therapy had to begin within 6 weeks of tissue diagnosis. All institutions obtained institutional review board approval prior to patient recruitment and all patients signed approved informed consents prior to trial enrollment.
Trial Design and Treatment
This was a phase III, randomized, multicenter, prospective trial. Under permuted block randomization,8 patients were stratified by age (<50 y vs ≥50 y), KPS (60–80 vs 90–100), and extent of surgery (biopsy only vs resection) and then randomly assigned to RT plus TMZ (RT/TMZ arm) or RT plus NU (RT/NU arm). NU therapy was either BCNU or CCNU.
RT was given in 1.8 Gy fractions, 1 fraction per day, 5 days per week to a dose of 59.4 Gy in 33 fractions. The initial 50.4 Gy in 28 fractions included the initial target volume (T2 abnormality plus 2-cm margin) or contrast-enhancing lesion + 2.5 cm when no T2 abnormality was present. The final 9 Gy in 5 fractions included the boost volume (T1-enhanced MR plus 1-cm margin). The target volumes received 95% to 105% of the prescribed dose. TMZ (200 mg/m2) was administered orally on days 1 through 5 of the first week of RT and then repeated every 28 days for a total of 12 cycles. BCNU (80 mg/m2) was administered intravenously on days 1, 2, and 3 of the first week of RT and on days 56, 57, and 58, and then every 8 weeks for 4 more cycles for a total of 6 cycles (maximum BCNU dose 1440 mg/ m2). The CCNU dose was 130 mg/m2 orally every 8 weeks for a total of 6 cycles. Concurrent therapy with corticosteroids and Pneumocystis carinii prophylaxis was allowed. Late and acute toxicities were graded using the RTOG/European Organisation for Research and Treatment of Cancer (EORTC) late morbidity scoring scheme and the National Cancer Institute Common Toxicity Criteria version 2, respectively. Treatment was stopped at disease progression or for unacceptable toxicity. At the discretion of the investigator, the patient could receive treatment with additional chemotherapy, biological therapy, surgery, or supportive care.
Evaluations
Baseline examinations included physical examination, brain imaging with MRI or CT, full blood cell counts and blood chemistry, chest x-ray, pulmonary function tests, and the Mini-Mental State Examination. Patients were seen weekly during radiotherapy and received a full clinical examination, blood hematology and chemistry tests, brain imaging, and pulmonary function tests prior to every cycle of chemotherapy. Tumor response and progression were defined according to Macdonald criteria9 and centrally confirmed. After completing treatment, patients were monitored clinically every 3 months in year 1, every 6 months in years 2 and 3, and annually thereafter.
Molecular Methods
IDH1-mutational status was determined by immunohistochemistry (IHC) with the mutation-specific monoclonal anti-IDH1-R132H antibody (dilution 1:50; Dianova) for 30 minutes at room temperature. Staining was performed on a Leica BOND RX auto stainer and included the Bond Epitope Retrieval Solution 1 (20 min) and the Bond Polymer Refine Detection Kit (Leica Biosystems). Tumor specimens were scored positive when tumor cell cytoplasmic staining for mIDH1-R132H was evident, whereas staining of macrophages was not scored positive. Because of the limited samples available for evaluation, analysis of IDH mutational status by sequencing was not performed.
Statistical Methods
The primary endpoint was OS. The hypothesized median survival time was 36 months for the RT/NU arm and 54 months for the RT/TMZ arm, corresponding to a hazard ratio (HR) of 0.67. It was projected that a sample size of 216 evaluable patients per arm would provide a 90% statistical power with a one-sided significance level of .05. When accrual rates were not met, statistical power was reduced from 90% to 80%, with the original hypothesized effect size on OS kept the same. The final analysis was planned after a total of 155 deaths were observed in the entire study cohort. A single interim futility analysis was performed when 127 of 155 deaths (82.0%) were observed, with an HR of 0.946 as the futility boundary.
OS was measured from the date of randomization to the date of death, or otherwise the last follow-up date on which the patient was reported alive. Progression-free survival (PFS) was measured from the date of randomization to the date of progression, death, or otherwise the last follow-up date on which the patient was reported alive without disease progression. OS and PFS were estimated using the Kaplan–Meier method, and distributions between the 2 treatment arms were compared using the Cox proportional hazards model and the log-rank test. Time to tumor progression (TTP), measured from the date of randomization to the date of progression, was estimated using the cumulative incidence function (CIF), with death without progression as a competing risk. The CIFs on tumor progression were compared between the 2 arms using a 2-sided test based on Gray’s method. Multivariate analyses were carried out on OS and PFS using the Cox proportional hazards model and on tumor progression using the Fine–Gray method, with patient baseline characteristics as covariates. Toxicity was compared between treatment arms using 2-sided Pearson chi-square tests.
The prognostic value of IDH1-R132H mutation status by IHC was investigated using the Cox proportional hazards model, with OS and PFS as the outcomes. The HRs on the effect of the biomarker were estimated and tested using 2-sided log-rank tests. Multivariate analyses were performed with patient baseline characteristics and the assigned treatment as covariates. A significance level of .05 was used in all the above-mentioned tests.
Results
Enrollment
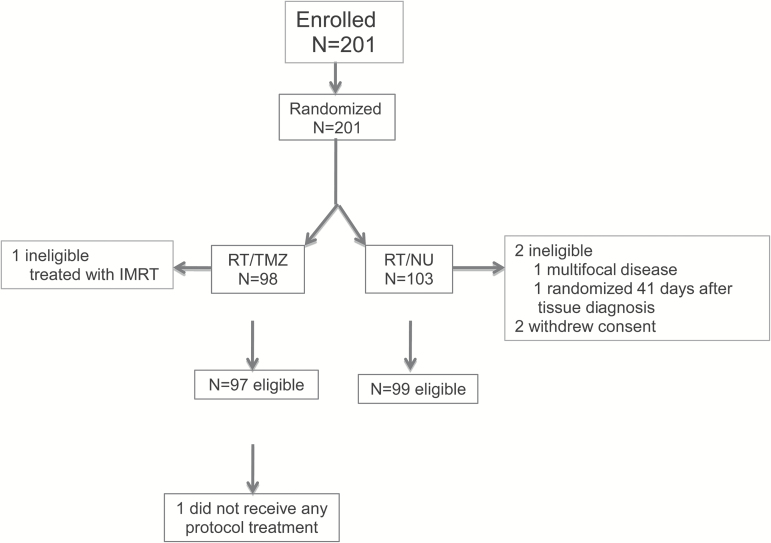
This study opened to accrual on October 15, 2002 and was temporarily closed to accrual between October 7, 2005 and April 6, 2006 due to a supply shortage of BCNU. The study was then amended to allow either CCNU or BCNU for the standard arm. The study was closed on March 30, 2007 because the accrual rate did not meet the target. A total number of 201 patients, accrued from institutions with institutional review board approval, were randomized, 98 to the RT/TMZ arm and 103 to the RT/NU arm. Overall, 3 patients (1.5%) were subsequently found to be ineligible, and 2 (1.0%) withdrew their consent (Fig. 1).
Fig. 1.
Consort diagram of patient disposition
Patient Characteristics
Table 1 lists pretreatment characteristics for all eligible patients by treatment arm. The distributions of all characteristics, including the stratification factors (age, KPS, and surgery at randomization), were balanced between the treatment arms.
Table 1.
Pretreatment characteristics and treatment compliance
| RT+TMZ (n = 97) | RT+NU (n = 99) | P d | |
|---|---|---|---|
| Age, y | |||
| Median | 42 | 43 | |
| Min–Max | 18–73 | 19–80 | |
| Q1–Q3a | 32–52 | 32–53 | |
| <50 | 67 (69.1%) | 68 (68.7%) | .99 |
| ≥50 | 30 (30.9%) | 31 (31.3%) | |
| KPS | |||
| 60–80 | 27 (27.8%) | 29 (29.3%) | .95 |
| 90–100 | 70 (72.2%) | 70 (70.7%) | |
| Surgery | |||
| Biopsy only | 37 (38.1%) | 34 (34.3%) | .69 |
| Resection | 60 (61.9%) | 65 (65.7%) | |
| Histology from central review | |||
| Anaplastic astrocytoma | 94 (96.9%) | 97 (98.0%) | .98 |
| Oligodendroglioma | 3 (3.1%) | 2 (2.0%) | |
| Radiation completed as per protocol | 92 (95.8%b) | 95 (96.0%) | .99 |
| Chemotherapy completed as planned | 58 (60.4% b) | 21 (21.4% c) | <.001 |
| Reasons for chemotherapy termination | |||
| Progression/death | 27 (28.1%b) | 38 (38.8%c) | |
| Side effect/toxicity | 0 (0.0% b) | 27 (27.6% c) | <.001 |
| Patient decision | 9 (9.4%b) | 6 (6.1%c) | |
a Ql = first quartile; Q3 = third quartile.
b One patient on the RT+TMZ arm did not receive any protocol treatment.
c One patient on the RT+NU arm did not receive any chemotherapy.
d Differences between the treatment arms were tested using the chi-square test.
Protocol Compliance
Compliance with the protocol-defined treatment plan was high for both radiation and chemotherapy. Radiation treatment parameters were acceptable in 89.7% of the RT/TMZ arm and 94.9% of the RT/NU arms. In the RT/TMZ arm there were 6 patients (6.2%) with unacceptable deviations, and 2 patients (2.1%) died during RT. In the RT/NU arm there were 4 patients (4.0%) with unacceptable deviations, and 1 patient (1.0%) progressed during radiation. Chemotherapy treatment was acceptable in 94.8% and 94.0% of the RT/TMZ and RT/NU arms, respectively. Table 1 summarizes treatment completion by arm. Chemotherapy was completed as planned for 60.4% of the patients in the RT/TMZ arm and 21.4% of those in the RT/NU arm. Chemotherapy was terminated due to progressive disease in 25.0% and 35.7% of the RT/TMZ and RT/NU arms, respectively. No patient in the RT/TMZ arm and 27.6% of the patients in the RT/NU arm discontinued chemotherapy because of side effects.
Safety and Long-Term Side Effects
Of the 195 (99.5%) eligible patients with toxicity information, those treated with NU had significantly more grade ≥3 toxicities than those treated with TMZ (75.8% vs 47.9%; P<.001). The majority of these toxicities were related to myelosuppression (Table 2). There were 2 grade 5 toxicities in both arms. The causes of death were myocardial ischemia and neutropenic infection in the RT/TMZ arm, and adult respiratory distress syndrome and pulmonary embolism in the RT/NU treatment arm.
Table 2.
Toxicity
| Worst Nonhematologic Toxicity by Treatment | ||
|---|---|---|
| RT+TMZ (n = 96) | RT+ NU (n = 99) | |
| Toxicity | ||
| Grade <3 | 65 (67.7%) | 65 (65.7%) |
| Grade ≥3 | 31 (32.3%) | 34 (34.3%) |
| P-value (chi-square test) = 0.76 | ||
| Worst Overall Toxicity by Treatment | ||
| RT+TMZ (n = 96) | RT+ NU (n = 99) | |
| Toxicity | ||
| Grade <3 | 50 (52.1%) | 24 (24.2%) |
| Grade ≥3 | 46 (47.9%) | 75 (75.8%) |
| P-value (chi-square test) < 0.001 | ||
Results for Overall Survival
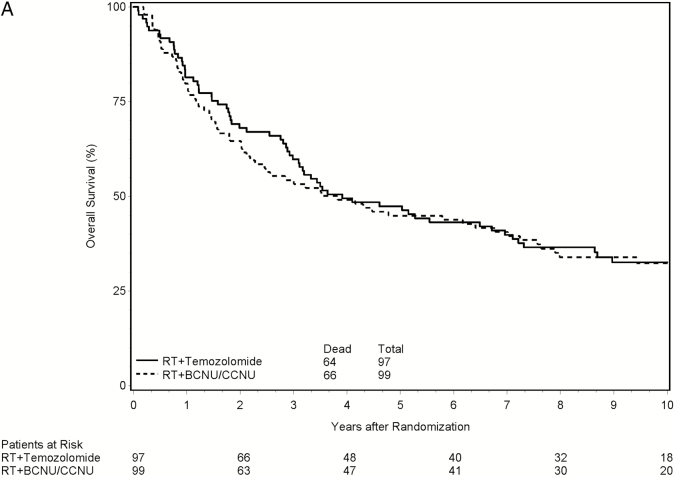
A decision was made to report the primary endpoint based on the results of the interim futility analysis. With 127 deaths observed from the 196 eligible and randomized patients, the observed HR on OS of the experimental arm relative to the standard arm was 0.957, which crossed the futility boundary at 0.946. At the time of this report, 130 patients (66.3%) had died. The median follow-up time for all 196 patients was 3.6 years (range: 0.1–12.6 y), and the median follow-up time for the 66 eligible patients that were still alive was 10.1 years (range: 1.9–12.6 y). Median survival time was 3.9 years in the RT/TMZ arm (95% CI, 3.0–7.0) and 3.8 years in the RT/NU arm (95% CI, 2.2–7.0), corresponding to an HR of 0.94 (P = .36; 95% CI, 0.67–1.32). In the multivariate analysis, the HR for the treatment effect on OS was 0.81 (P = .24; 95% CI, 0.57–1.15 years). Figure 2A shows the Kaplan–Meier curves for OS by treatment arm.
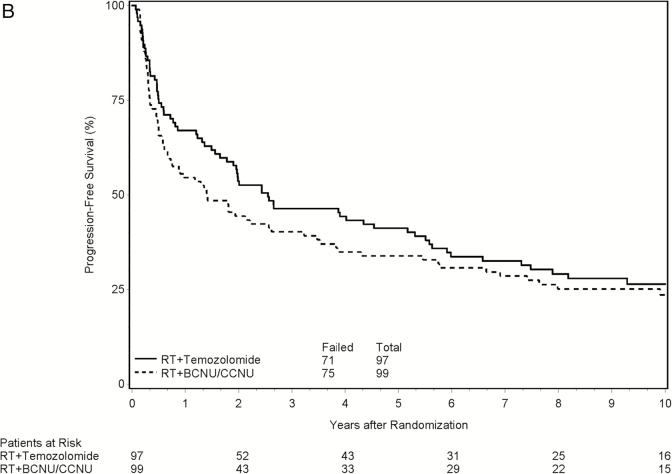
Fig. 2.
Clinical outcomes by treatment
Results for Secondary Endpoints
For PFS, the observed HR on the treatment effect was 0.85 (P = .31; 95% CI, 0.61–1.17) from the univariate analysis. After adjusting for the stratification factors and other pretreatment characteristics, the treatment effect was found to be statistically significant with HR = 0.70 (P = .039; 95% CI, 0.50–0.98 y) favoring the TMZ arm. No significant difference for TTP between the 2 treatment arms was shown on either univariate analysis (P = .46) or multivariate analysis (P = .24; HR = 0.80; 95% CI, 0.55–1.16). Figure 2B shows the Kaplan–Meier curves for PFS by treatment arm.
Correlative Biomarker Studies
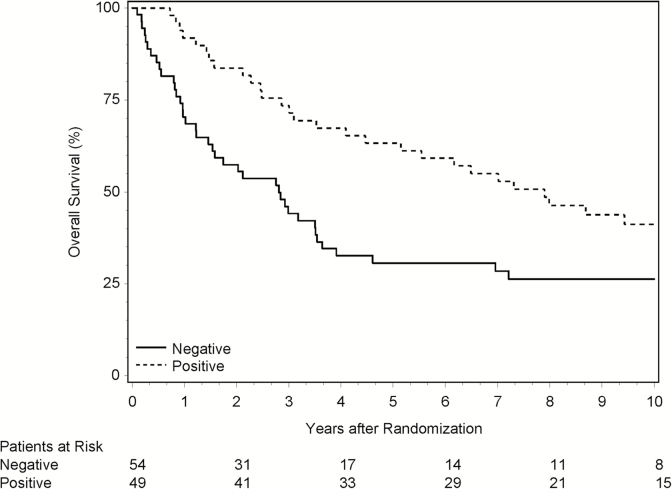
Out of the 196 eligible and randomized patients, 111 were tested for IDH1-R132H mutation status by IHC, 60 on the RT/TMZ arm, and 51 on the RT/NU arm. There were 8 patients (7.2%) whose IDH1 status could not be scored, and they were excluded from subsequent analyses. Overall, 54 patients (48.6%) were IDH1-R132H mutation negative by IHC and 49 (44.1%) were positive (Table 3). More patients with positive status had favorable pretreatment characteristics related to age (<50 y), KPS (90–100), and surgery (resection). In the univariate analyses, IDH1 mutation positive appeared to be associated with improved OS (HR = 0.50; P = .004; 95% CI, 0.31–0.81) and PFS (HR = 0.59; P = .02; 95% CI, 0.37–0.92). The prognostic value of IDH1 mutation was confirmed in the multivariate analyses with the stratification factors included as covariates, with HRs of 0.42 (P = .001; 95% CI, 0.25–0.72) for OS and 0.53 (P = .010; 95% CI, 0.32–0.86) for PFS. Figure 3 depicts the Kaplan–Meier curves for OS based on IDH1 mutation status.
Table 3.
IDH1-R132H mutation status
| RT+TMZ | RT+ NU | Total | ||||
|---|---|---|---|---|---|---|
| IDH1-R132H mutation | n | % | n | % | n | % |
| Negative | 31 | 51.7 | 23 | 45.1 | 54 | 48.6 |
| Positive | 24 | 40.0 | 25 | 49.0 | 49 | 44.1 |
| Not scored | 5 | 8.3 | 3 | 5.9 | 8 | 7.2 |
| Total | 60 | 100.0 | 51 | 100.0 | 111 | 100.0 |
IDH, isocitrate dehydrogenase.
IDH1-R132H mutation status was tested in 111 of 196 eligible patients.
Fig. 3.
Overall survival by IDH1-R132H mutation status
Discussion
This is the first study to exclusively compare the OS of patients with grade III glioma with astrocytic-dominant histology treated with radiation and nitrosourea versus radiation and temozolomide. A major strength of the study is that it addressed an important question regarding 2 cytotoxic regimens with radiation in a group of patients for whom histologic criteria for eligibility were centrally reviewed. Protocol treatment compliance was very high and in a subset of patients important correlative biomarker studies were explored. Although the results showed equivalent OS and TTP between the 2 regimens, toxicity was noted to be significantly higher in the nitrosourea arm; 28% of patients in the NU arm discontinued chemotherapy because of side effects and no patients in the TMZ arm were removed from study because of toxicity. The high frequency of grade 3 or higher toxicity in the RT/TMZ arm may be reflective of the different dose schedule with concurrent radiation as was given in the Stupp regimen.6
RT/TMZ did not appear to significantly improve OS or TTP compared with RT/NU, suggesting that the type of alkylating agent may not be a major factor for efficacy. The NOA-04 study randomized 274 patients with anaplastic glioma (52.6% anaplastic astrocytoma and 47.4% anaplastic oligodendroglioma) to RT alone versus either PCV or TMZ alone at the time of initial treatment, followed by cross-over at relapse, and long-term results did not demonstrate a significant survival difference in any favor of any of the arms,10 also implying possible therapeutic equivalence, with lower toxicities for the TMZ arm. On the same basis of comparable outcome with lower toxicity, our study would also support the use of TMZ over NU for newly diagnosed anaplastic astrocytoma.
The approved TMZ regimen at the time of the study design was the 5-day/28-day regimen. The regimen demonstrating a survival advantage in glioblastoma had a concurrent RT phase with daily dosing of TMZ followed by an adjuvant 5/28 regimen.6 It is unknown if this regimen would have resulted in a survival benefit in our study or if the same survival advantage would have been seen in the glioblastoma study if an NU were used as the standard arm. A large international trial, CATNON, is being conducted in patients with newly diagnosed grade III glioma. Patients with anaplastic gliomas with intact 1p19q are randomized to radiation with or without temozolomide as per the Stupp regimen; following radiotherapy there is a second randomization to adjuvant temozolomide or not. This trial, which recently completed accrual, will address not only the benefit of adjuvant TMZ to RT in anaplastic astrocytoma but also the role of concurrent and adjuvant TMZ. A preplanned interim analysis demonstrated a statistically significant reduction in the overall death hazard for the use of maintenance TMZ, whereas there had not been sufficient events to comment on the role of concomitant TMZ.11
The primary limitation of this study was its early closure due to poor accrual rates. During the accrual phase, a randomized trial of TMZ with RT versus RT alone for newly diagnosed glioblastoma demonstrated a survival advantage that resulted in full approval of the agent in this setting in 2005.6 The ease of administration, the favorable toxicity profile, and status as the new standard of care for glioblastoma resulted in the wide adoption of RT with TMZ in the treatment of all types of high-grade glioma. This reflects the negative effect that a change in treatment recommendation for a related disease process can have on a trial’s successful completion. This will remain a challenge for any disease for which the duration of the study spans years, either for accrual because of low incidence rates or follow-up because of long survival times. This has been seen in studies of anaplastic oligodendroglioma and low-grade glioma, for which final survival analyses were reported more than 14 years after accrual was completed.12–14 Alternative, robust surrogate endpoints are needed in these instances, a major challenge in neuro-oncology. Response rates and TTP have inherent limitations and have yet to be validated against survival in many instances. Other limitations of this study were lack of neurocognitive or quality-of-life evaluations and the treatment regimens in both the TMZ and NU arms.
Since the initiation of the RTOG 9813 study, critical information on molecular and cytogenetic markers has changed our understanding of glioma classification, challenging the value of histologic assessment.15,16 Results of randomized studies in anaplastic oligodendroglioma13,14 clearly demonstrate the importance of 1p19q codeletion as a predictive marker, and other studies have confirmed the prognostic value of IDH mutations, as shown in a subset of patients in this study across both treatment arms. Results of the NOA-04 trial showed that hypermethylation of the MGMT promoter region, IDH1 mutations, and oligodendroglioma histology were associated with a decreased risk of progression and that IDH1 mutation was a more valuable prognostic factor for patients with anaplastic glioma than 1p19q codeletion or MGMT methylation status.10 In a separate report, the results of hypermethylation of MGMT and other molecular markers such as ATRX mutation status in patients treated on this study will be analyzed. The ability of these markers to better predict outcome has significant ramifications for the care of patients and design of clinical trials.
Conclusions
Although the study was closed early, RT/TMZ did not appear to significantly improve OS or PFS for anaplastic astrocytoma as compared with RT/NU. RT/TMZ was better tolerated. Molecular diagnostic criteria provide valuable prognostic information and need to be incorporated in the design of future studies.
Funding
This project was supported by grants U10CA21661 (RTOG-Ops-Stat), U10CA37422 (CCOP), U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC) from the National Cancer Institute (NCI) and Merck & Co. Grant funding for Correlative Studies: Ohio State University Comprehensive Cancer Center (to A.C.), R01CA108633 (to A.C.), 1RC2CA148190 (to A.C. and WC), 1R01CA169368 (to A.C.), NCI-CTEP #NRG-BN-TS002 (to EB and A.C.).
Conflict of interest statement. Dr. Chang reports consulting/advisory role with Agios Pharmaceuticals, Blaze, Edge, Neu-Onc and Tocagen and research funding to her institution from Agios Pharmaceuticals, Quest, Novartis, Roche, and Tocagen. Dr. Bell reports a patent or intellectual property interest with Ohio State University. Dr. Schiff reports honoraria from Merck, consulting/advisory role with Celldex, Genentech, Heron, Midatech Pharma, Oxigene, and VBI and his institution has a consulting advisory role with Cavion. Dr. Jaeckle reports consulting/advisory role with Orbus Therapeutics and Bristol-Myers Squibb and travel expenses from BMS. Dr. Shih reports consulting/advisory role with Genentech. Dr. Brachman reports a patent or intellectual property interest in GammaTile. Dr. Penas-Prado reports research funding to her institution from Abbvie, Agios Pharmaceuticals, Arog Pharmaceuticals, Genentech, GSK, Merck, and Novartis. Dr. Schultz reports research funding to his institution from Elekta AB and Phillips Healthcare. Dr. Mehta reports grants and personal fees from Novocure and Novelos, personal fees from Abbott, Phillips, BMS, Celldex, Roche, Elekta, Novartis, Cavion, Pharmacyclics, Monteris, and Varian.
Acknowledgments
We would like to acknowledge Andrea Salavaggione, MD, for her assistance with the IDH1 immunohistochemistry assays and Ilona Garner, University of California, San Francisco, for editing the manuscript.
References
- 1. Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. [DOI] [PubMed] [Google Scholar]
- 2. Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19(2):509–518. [DOI] [PubMed] [Google Scholar]
- 3. Prados MD, Scott C, Curran WJ, Jr, et al. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: a retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999;17(11):3389–3395. [DOI] [PubMed] [Google Scholar]
- 4. Bower M, Newlands ES, Bleehen NM, et al. Multicentre CRC phase II trial of temozolomide in recurrent or progressive high-grade glioma. Cancer Chemother Pharmacol. 1997;40(6):484–488. [DOI] [PubMed] [Google Scholar]
- 5. Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17(9):2762–2771. [DOI] [PubMed] [Google Scholar]
- 6. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 7. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27(7-8):365–375. [DOI] [PubMed] [Google Scholar]
- 9. Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 10. Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 11. Van den Bent MJ, Erridge S, Vogelbaum MA, et al. Results of interim analysis of the EORTC randomized phase III CATNON trial on concurrent and adjuvant temozolomide in anaplastic glioma without 1p/19q co-deletion: An Intergroup trial. J Clin Oncol. 34,2016. (suppl; abstr LBA2000). [Google Scholar]
- 12. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus Procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 15. Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]