Figure 1.
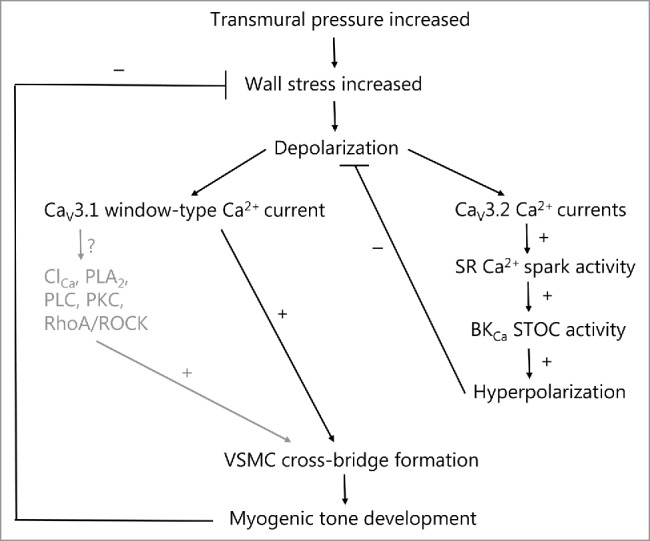
T-type calcium channels in spontaneous myogenic tone development in the rat middle cerebral artery and mouse small mesenteric artery, as explained in the text. The well-established role of L-type calcium channels is omitted here. At VSMC membrane potentials more hyperpolarized than the activation threshold for L-type channels, increases in pressure in the low range from 40–80 mmHg leads to low voltage-activation of CaV3.1 and/or CaV3.2 T-type calcium channels. The activation of CaV3.1 in this pressure range leads to myogenic tone development either via low sustained window-type Ca2+ currents, or as proposed here, via subsequent activation of Ca2+-dependent signaling molecules in membrane micro-domains (caveolae). The activation of CaV3.2 channels leads to Ca2+-dependent activation of RyRs in closely apposed SR causing increased Ca2+ spark activity. This in turn will activate nearby BKCa channels to increase STOC activity leading to hyperpolarization of VSMCs, which causes a negative feedback on the pressure-dependent depolarization and on spontaneous myogenic tone development. Thus activation of T-type channels at lower pressures may cause both an activation as well as an inhibition of myogenic tone, and the balance between the 2 opposing roles presumably relies on the vascular bed and vessel diameter. Text shown in gray are proposed signaling mechanisms that are so far unexplored. Stimulation and inhibition of an activity is shown as + or −, respectively. SR (sarcoplasmic reticulum); BKCa (large-conductance calcium-activated potassium channel); STOC (spontaneous transient outward current), ClCa (calcium-activated chloride channel, such as TMEM16A); PLA2 (Ca2+-dependent cytosolic phospholipase A2); PLC (calcium-dependent phospholipase C-δ); PKC (protein kinase C-α/β); RhoA/ROCK (small G-protein RhoA/Rho-kinase pathway).
