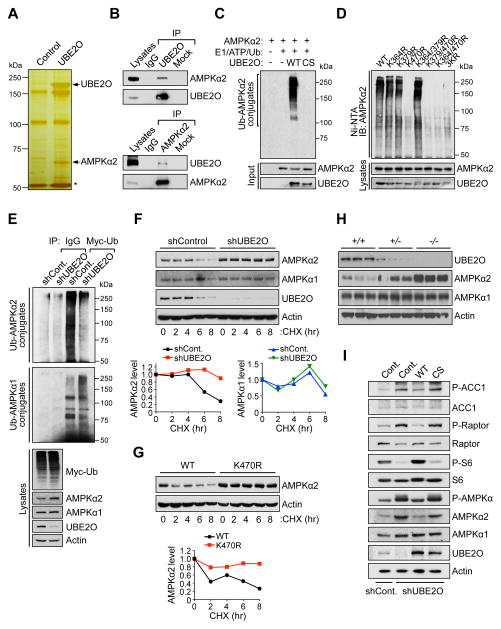
Figure 3. UBE2O specifically targets AMPKα2 for ubiquitination and degradation.
(A) Silver stained gel of proteins recovered after immunoprecipitation of lysates prepared from Ube2o−/− MEFs expressing Flag-tagged UBE2O. Proteins captured with anti-Flag were eluted with Flag peptide. Shown are proteins present in the Flag eluate. Asterisk indicates the heavy chain of IgG.
(B) Lysates from HeLa cells were immunoprecipitated (IP) with IgG and anti-UBE2O (top) or anti-AMPKα2 (bottom) then immunoblotted as indicated.
(C) Recombinant AMPKα2 proteins were subjected to in vitro ubiquitination assay in the presence of in vitro-translated wild-type (WT) or C1040S (CS) mutant UBE2O.
(D) Lysates from Prkaa1−/−Prkaa2−/− MEFs expressing WT or various indicated lysine-to-arginine (KR) mutants of AMPKα2 treated with MG132 (10 μM) for 4 hr were subjected to metal-affinity purification for His-tagged ubiquitin then immunoblotting for ubiquitinated AMPKα2. Ni-NTA, Ni2+-nitrilotriacetic acid.
(E) Lysates from HCT116 cells expressing UBE2O shRNA together with Myc-tagged ubiquitin treated with MG132 (10 μM) for 4 hr were immunoprecipitated (IP) with IgG or anti-Myc and analyzed for ubiquitination with anti-AMPKα2 or anti-AMPKα1.
(F) Lysates from HCT116 cells expressing UBE2O shRNA treated with cycloheximide (CHX) for the indicated times were subjected to immunoblotting (top). AMPKα2 or AMPKα1 protein levels were quantified by normalizing to the intensity of the Actin band (bottom).
(G) Lysates from Prkaa1−/−Prkaa2−/− MEFs expressing WT or K470R mutant AMPKα2 treated with CHX for the indicated times were subjected to immunoblotting (top). AMPKα2 protein levels were quantified by normalizing to the intensity of the Actin band (bottom).
(H) Lysates from mammary tissues of 13 weeks old Ube2o+/+, Ube2o+/− and Ube2o−/− mice were subjected to immunoblotting.
(I) Lysates from primary MEFs expressing UBE2O shRNA together with WT or CS mutant UBE2O were subjected to immunoblotting.
See also Figures S2 and S3.