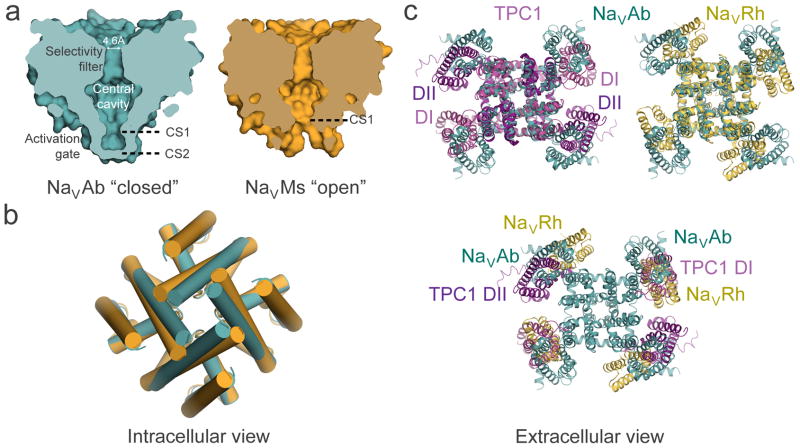
Figure 4. Structural model of conformational changes during channel activation and pore opening.
a, Pore-opening conformational change between the closed pore of NaVAb and the open pore of NaVMs. NaVAb structure (left, cyan) contains two pore constriction sites (CS1 and CS2) with CS2 site completely sealing the pore. NaVMs structure (right, orange) contains one pore constriction site (CS1) that remains open to allow hydrated sodium ion to pass through. b, Superposition of NaVAb and NaVMs pores viewed from the intracellular side. A counterclockwise twisting motion of the S6 segment from the closed pore of NaVAb to the open pore of NaVMs shifts the end of S6 helix outward to dilate the pore diameter. c, Channel activation involves clockwise rotation of the voltage sensor around the pore. Top left: Superposition of TPC1 (light purple for activated state domain I (DI) and dark purple for resting state domain II (DII)) and NaVAb (cyan) structures. Top right: Superposition of NaVRh (yellow) and NaVAb (cyan) structures. Bottom: Overlay of voltage-sensing modules in TPC1, NaVAb, and NaVRh as in the top panel but with the pore modules of TPC1 and NaVRh omitted for clarity. The voltage-sensing module progressively rotates around the pore from the most resting state in TPC1 DII to increasingly activated states in NaVAb, TPC1 DI, and NaVRh.