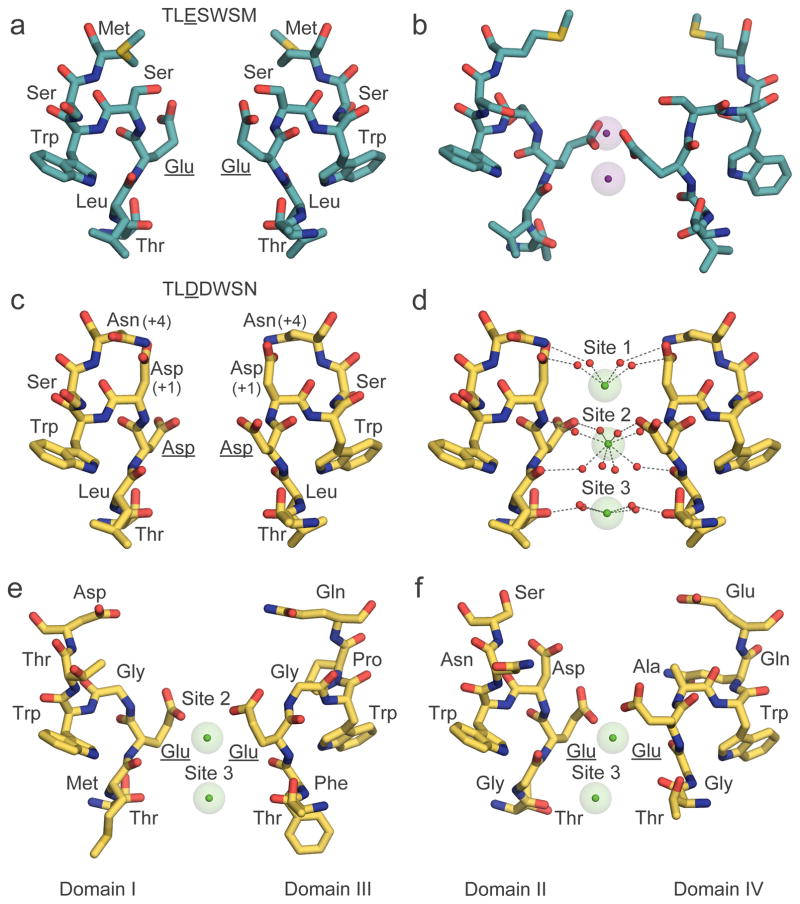
Figure 5. Chemical mechanism of ion permeation and selectivity of NaV and CaV channels with structural models of their ion selectivity filters with ions bound.
a, Na+ selectivity filter (TLESWSM) in NaVAb. b, Representative conformations of Na+ selectivity filter from molecular dynamic simulations of sodium permeation in NaVAb. Conformational dunking of Glu side chain of the high-field strength site allows direct coordination of Na+ ions. c, Ca2+ selectivity filter (TLDDWSN) in CaVAb. d, Hydrated Ca2+ bound in the CaVAb selectivity filter. e, Ca2+ selectivity filter of CaV1.1 from Domains I (TMEGWTD) and III (TFEGWPQ). f, Ca2+ selectivity filter of CaV1.1 from Domains II (TGEDWNS) and IV (TGEAWQE). Na+ (purple) and Ca2+ (green) ions are shown with semi-transparent ionic sphere. Dash lines indicate network of interactions among coordinated water molecules with the ions and protein atoms from high-filed strength site (Glu in NaVAb and CaV1.1, and Asp in CaVAb, underlined) and backbone carbonyls of Leu and Thr. For clarity, only two opposing subunits in the tetramer are shown. Of note, a distantly related non-voltage-gated Ca2+ channel has a different architecture of its outer pore with Asp residues from each subunit directly binding dehydrated Ca2+ in a closed state structure56. The significance of this binding mode in ion conductance is unknown.