Figure 1.
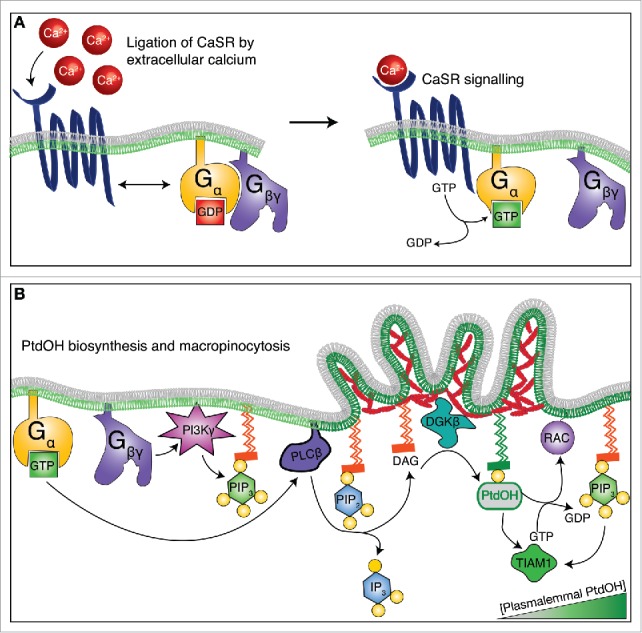
The growth factor-independent macropinocytosis and membrane ruffling of professional phagocytes is driven by extracellular calcium. (A) In response to extracellular calcium, the 7-transmembrane receptor CaSR changes conformation and catalyzes nucleotide exchange on its coupled Gα protein. The Gβγ subunit then dissociates from Gα-GTP, but each component of the complex gains signaling functions. (B) Active (GTP-bound) Gα activates PLC, which in turn mediates the breakdown of PtdIns(4,5)P2 into DAG and Ins(1,4,5)P3. In parallel, the Gβγ subunit promotes the formation of PtdIns(3,4,5)P3 by activating PI3K. The formation of DAG is rapidly followed by its phosphorylation by DGK into PtdOH, which serves multiple signaling roles in membrane ruffling and macropinocytosis. In particular, PtdOH recruits GEFs that carry polybasic amino acid clusters and activate the small GTPase RAC1/2. Following its activation, RAC1/2 catalyzes the nucleation of the branched actin networks that propel membrane ruffles and macropinosome formation.
