Abstract
Background.
Meningiomas are the most common primary intracranial tumors in adults. Identification of SMO and AKT1 mutations in meningiomas has raised the hope for targeted therapies. It would be useful to know the precise frequency of these mutations in anatomical subgroups and clarify their prognostic value.
Methods.
We used the Sanger sequencing technique to characterize 79 samples of olfactory groove meningiomas for SMO (L412F and W535L) and AKT1E17K mutations. We reviewed clinical data to assess the prognostic value of these mutations in this anatomical subgroup.
Results.
Out of the 79 patients with olfactory groove meningiomas, we identified targetable mutations in 34 patients (43%) (22 patients [28%] with SMO mutation—L412F almost exclusively—and 12 patients [15%] with AKT1 mutation). Meningiomas in the SMO-mutant group had an overall 36% recurrence rate, significantly higher than in the AKT1-mutant group (16%) and in the “SMO and AKT1 wildtype” group (11%) (χ2 test, P = .04). All late recurrences (after 5 y) occurred in the SMO-mutant group. Among grade I meningiomas, the SMO-mutant group was identified as having a significantly poorer prognosis. World Health Organization histological grade II (P = .006) and incomplete resection (P = .001) were independently associated with shorter recurrence-free survival.
Conclusion.
Molecular diagnosis of SMOL412F/W535L and AKT1E17K mutations improves prognostic evaluation in olfactory groove meningiomas and opens new therapeutic perspectives with SMO or AKT inhibitors for recurrent cases.
Keywords: AKT, meningioma, molecular pathology, olfactory groove meningioma, SMO
Importance of the study
We analyzed a large series of olfactory groove meningiomas for SMO and AKT1 mutations, 2 targetable missense mutations previously described in meningiomas. We identified a SMO mutation (L412F or W535L) in 28% and an AKT1E17K mutation in 15% of our cases. The high frequency of SMO mutations in meningiomas arising from the anterior and medial skull base might be explained by the central role of the sonic hedgehog pathway in craniofacial development during embryogenesis. In addition, time-to-recurrence analysis shows that among grade I skull base meningiomas, the group with SMO mutations had a significantly poorer prognosis. Our study strongly supports the systematic screening of these prognostic markers and potentially actionable targets in skull base meningioma.
Meningiomas are the most common primary brain tumors in adults.1 Olfactory groove meningiomas (OGM) represent 4% to 13% of all intracranial meningiomas2 and originate from the meningeal cells covering the lamina cribrosa and the planum sphenoidale. Their extent can damage frontal lobes and olfactory and visual functions.
Despite excellent tumor control in most cases after surgery,2,3 recurrence occurs in 5% to 30% according to follow-up (FU) duration.4–6 Prognostic factors identified in OGM are age, World Health Organization (WHO) grade, and extent of resection.4,7
Removal of large or invasive tumors may be associated with significant visual and cognitive morbidity, particularly at relapse.8 At recurrence, treatment options include follow-up if the tumor growth is minimal, total resection if feasible, or radiosurgery/therapy.8,9 In rare cases, refractory meningiomas can invade the anterior skull base and/or paranasal sinuses with severe visual impairment and can be life threatening. To date, medical therapies have been largely ineffective in those cases.10
Biallelic inactivation of NF2 is the most frequent genetic event in meningioma tumorigenesis.11–13 However, anterior skull base topography is strongly associated with intact NF2 gene status and meningothelial histological subtype.14,15
Recently, driver mutations in TRAF7, KLF4, PI3KCA, POLR2A, AKT1, and SMO genes have been identified in non–NF2-mutant meningiomas.14,16–18 All AKT1-mutant meningiomas (12% of all meningiomas) harbored the same activating mutation (E17K) and were associated with lesions of low malignancy potential located at the anterior or middle skull base.19–21 The SMO-mutant meningiomas described in the literature (with a frequency between 3% and 5% of all meningiomas) showed 2 recurrent variants (L412F [70%] and W535L [15%]) and a highly recurrent phenotype of grade I meningothelial lesions arising from the anterior medial skull base.16,20 Interestingly, therapies are available specifically targeted against these 2 oncogenic drivers: a multicenter phase II study of vismodegib and afuresertib is under way for patients with progressive or recurrent SMO- and AKT1-mutant meningiomas, respectively (NCT02523014, clinicaltrials.gov).
Here, we report the first molecular analysis of SMO and AKT1 mutations in a large OGM series, with particular emphasis on prognosis and recurrence rate.
Patients and Methods
Patient and Tissue Samples
Collection of patient samples and clinico-pathological information was undertaken with patient informed consent and hospital ethical board approval. We requested the following data from our Brain Tumor Bank (Onconeurothèque): histological diagnosis of meningioma and surgery between 2003 and 2012. We reviewed the following parameters for all patients: sex, age, Karnofsky performance scale (KPS), presenting symptoms, radiological features, extent of surgical resection and complications, treatment modalities (number of surgical procedures, need for radiotherapy or radiosurgery), and recurrence (site, time to recurrence). Exact topography on MRI and radiological features were centrally reviewed to ensure that “olfactory groove” topography was present (ie, lamina cribrosa and planum sphenoidale). The extent of resection was determined according to the Simpson grade system.22 A central pathology review was carried out by our senior pathologist (F.B.) in order to validate histological features (subtype and grading according to WHO 2016 classification, Ki67 proliferation index).
Follow-up
Patients were followed up with clinical examination and MRI at 6 months and 1 year after surgery. Then they were followed regularly every 1 to 2 years. Tumor recurrence was defined as an increase in residual tumor or the reappearance of onsite lesions on FU imaging (MRI in all cases).
Molecular Characterization
DNA extraction
DNA was extracted from fresh-frozen tumors using the QIAmp DNA minikit (Qiagen) and quantified using Nanodrop (Thermo Fischer Scientific).
Sanger sequencing
Sequencing for SMO was restricted to recurrent L412F and W535L mutations, with primers as follows: SMOL412F_F: AGTGACTGGTAGGAACGGGAG; SMOL412F_R: GGAGCTAGCTGGGGTTTCAG; SMOW535L_F: CCCATCCCTGACTGTGAGAT; SMOW535L_R: CAGGTACGCCT CCAGATGAG.
Fragments spanning codon 17 of AKT1 were amplified using the following primers: AKT1E17K_F: AAGACTCGGCAGCATCTCCAGCAGGCATCCCAGGCACA TCTGTCCAKT1E17K_R: GCGATCGTCACTGTTCTCAGTA GCGTGGCCGCCAGGTCTTGATGTACT.
PCR was carried out with a total volume of 10 μL containing 50 ng template DNA in Fast Start PCR Master Mix (Roche). Initial denaturation at 94°C for 10 min was followed by 30 cycles with denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min, followed by 10 min at 72°C. Then, 3 μL of amplification product was submitted to sequencing (Genoscreen). Sequences were read with 4Peaks software (Macintosh).
Statistical Analysis
The χ2 test (with Yates correction when appropriate) and the Kruskal–Wallis test were used to compare the distributions of categorical and continuous variables, respectively. Progression-free survival (PFS) was defined as the time between surgery and recurrence or last FU. Patients who were recurrence free at last FU were considered to be censored in analysis. Kaplan–Meier survival curves were plotted, and differences in survival between groups of patients were compared with the log-rank (Mantel–Cox) test. The influence of age (<70 vs ≥70 y), Simpson grade (I–II vs III–IV), WHO histological grade (I vs II), and mutational status on SMO and AKT1 genes in recurrence-free survival were assessed. Multivariate analysis was then performed with the Cox proportional hazards model incorporating the major prognostic factors of univariate analysis. All statistical tests were 2-sided and a P-value of ≤.05 was considered to be statistically significant. Statistical analyses were performed using Statview version 5.0 software (SAS Institute).
Results
Patient Population
Among the 1439 frozen meningioma samples available in Onconeurothèque, 260 were described as “anterior skull base” meningiomas (orbital, olfactory groove [including lamina cribrosa and planum sphenoidale], tuberculum sellae, anterior clinoid process, or suprasellar meningiomas). Among them, 79 were confirmed as olfactory groove tumors based on surgical and radiological data and were included in our study: the main demographic characteristics are summarized in Table 1. There were 62 females (79%) and 17 males (21%). Median preoperative KPS was 90 (range, 40–100); 76 patients were operated on for a primary tumor, and 3 for a recurrent tumor (data from the first surgery [pathology, Simpson grade, and PFS] were considered for these patients). Median FU was 70 months (range, 2–312). One patient died of a pulmonary embolism after surgery, and 5 patients (6.3%) had no reliable postoperative FU (one in the SMO group and 4 in the “others” group with neither SMO nor AKT1 mutation). Survival analysis was thus conducted on 73 patients who had at least one postoperative examination and imaging available.
Table 1.
Clinical and imaging data
| Demographic Data | General Cohort (n = 79) | SMO (n = 22) | AKT1 (n = 12) | Others (n = 45) | P |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 17 (21%) | 4 (18%) | 2 (16%) | 11 (25%) | .76 |
| Female | 62 (79%) | 18 (82%) | 10 (84%) | 34 (75%) | |
| Age, y | |||||
| Median | 55 | 55 | 56 | 55 | .94 |
| Range | 22–83 | 22–72 | 30–75 | 26–83 | |
| Median preoperative KPS | 90 | 90 | 80 | 90 | .95 |
| Median FU (mo) | 70 | 83 | 60 | 72 | .18 |
| Presenting symptoms | |||||
| Headache | 46 (58%) | 12 (54%) | 6 (50%) | 28 (62%) | .68 |
| Visual impairment | 33 (41%) | 13 (59%) | 3 (25%) | 17 (38%) | .11 |
| Anosmia | 56 (71%) | 20 (91%) | 7 (58%) | 29 (64%) | .04 |
| Cognitive impairment | 38 (48%) | 13 (59%) | 5 (42%) | 20 (45%) | .47 |
| Seizures | 4 (5%) | 2 (9%) | 1 (8%) | 1 (2%) | .41 |
| Imaging data | |||||
| Size | |||||
| Mean tumor diameter (mm) | 59 | 63 | 54 | 51 | .28 |
| Range | 30–90 | 35–80 | 30–90 | 30–70 | |
| Hyperostotic bone | 52 (66%) | 17 (77%) | 6 (50%) | 29 (65%) | .26 |
Molecular Subgroups
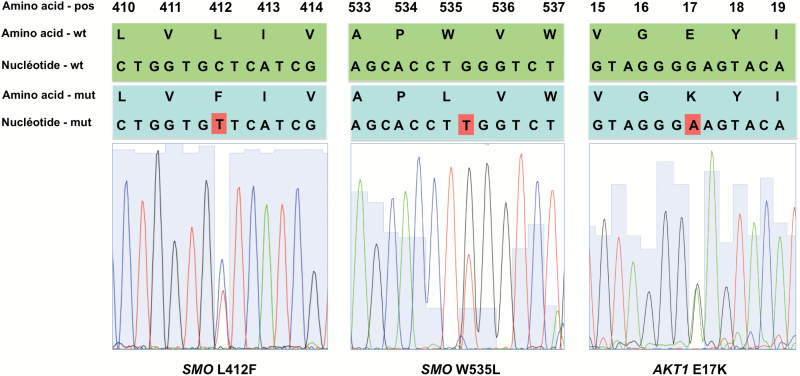
Of the 79 OGM, 22 (28%) carried a SMO mutation: 21 with L412F and 1 with W535L. Twelve (15%) presented an AKT1E17K mutation (Fig. 1). SMO and AKT1E17K mutations were mutually exclusive and no tumor had both mutations. The remaining 45 meningiomas were defined as “others” in a third group with neither SMO nor AKT1 mutation. Only one patient had a molecular analysis of a primary and a recurrent frozen meningioma that both contained SMO mutations.
Fig. 1.
Representative electropherograms of SMO L412F (left), SMO W535L (center), and AKT1 E17K (right) mutations.
Demographic, Clinical, and Perioperative Data
Demographic, clinical, and imaging findings in the 3 groups (SMO-mutant, AKT1-mutant, and “others”) are summarized in Table 1. There was no difference in demographic features among the 3 molecular subgroups. Presenting symptoms were similar in all groups, apart from anosmia, which was significantly more common in the SMO-mutant group (P = .01).
No significant difference was found in tumor size among the 3 groups. Peritumoral frontal edema on fluid attenuated inversion recovery–weighted sequences was present in all cases. Clinical and demographic findings from the general cohort were consistent with published OGM cohorts.2,4,5 Simpson grades I–II resection was achieved in 91%, 75%, and 88% of the SMO, AKT1, and “others” groups, respectively (P = .36) (Table 2).
Table 2.
Treatment modalities and recurrence rates
| Treatment Modalities Recurrence Rates |
General Cohort | SMO | AKT1 | Others | P |
|---|---|---|---|---|---|
| Simpson grade | |||||
| I–II | 69 (87%) | 20 (91%) | 9 (75%) | 40 (88%) | .36 |
| III–IV | 10 (13%) | 2 (9%) | 3 (25%) | 5 (12%) | |
| N surgery(ies) | |||||
| 1 | 72 (91%) | 18 (81%) | 11 (91%) | 43 (96%) | .17 |
| 2 | 5 (6%) | 3 (15%) | 1 (9%) | 1 (2%) | |
| ≥3 | 2 (3%) | 1 (4%) | 0 (0%) | 1 (2%) | |
| Radiotherapy/radiosurgery | 3 (4%) | 2 (9%) | 1 (8%) | 0 | .22 |
| Recurrence rate | 13 (16%) | 8 (36%) | 2 (16%) | 5 (11%) | .04 |
| 5 y PFS | 81% | 81% | 83% | 82% | .93 |
| 10 y PFS | 65% | 52% | 83% | 82% | .008 |
| Median PFS (mo) | 65 | 79 | 53 | 63 | .65 |
Comparative Analysis of Histological Features and Molecular Subgroups
The SMO-mutant meningiomas showed a uniform histological phenotype: all were WHO grade I and meningothelial, with low Ki67 index and 1 or less mitosis per 10 high-power fields. The AKT1-mutant meningiomas were more heterogeneous: 8 (67%) were grade I (all meningothelial) and 4 (33%) were grade II (one chordoid and 3 atypical). Other histological features are summarized in Table 3.
Table 3.
Histological features
| Pathology | General | SMO | AKT1 | Others | P |
|---|---|---|---|---|---|
| Grade | |||||
| I | 69 (89%) | 22 (100%) | 8 (67%) | 39 (89%) | .14 |
| II | 9 (11%) | 0 (0%) | 4 (33%) | 5 (11%) | |
| Ki 67 | |||||
| Mean | 4 | 3.55 | 4.18 | 4.45 | .22 |
| Range | 1–8 | 1–6 | 3–6 | 1–8 | |
| Histological subtype | |||||
| Meningothelial | 55 (69%) | 22 (100%) | 8 (67%) | 25 (55%) | .0004 |
| Fibroblastic | 4 (5%) | 0 | 0 | 4 (9%) | |
| Transitional | 7 (9%) | 0 | 0 | 7 (15%) | |
| Psammomatous | 2 (3%) | 0 | 0 | 2 (4%) | |
| Microcystic | 1 (1%) | 0 | 0 | 1 (2%) | |
| Atypical | 9 (11%) | 0 | 3 (25%) | 6 (13%) | |
| Chordoid | 1 (1%) | 0 | 1 (8%) | 0 | |
Impact of Molecular Characterization on Natural Course and Recurrence Rates
The main treatment modalities (surgery and radiotherapy; no patient was treated with systemic therapy) and recurrence rates are shown in Table 2. Meningiomas in the SMO-mutant group had an overall 36% recurrence rate, significantly higher than in the AKT1-mutant group (16%) and the “others” group (AKT1-wildtype, SMO-wildtype) (11%) (P = .04). Despite the fact that the 5-year PFS rate was similar in the 3 groups (81%, 83%, and 82%), the 10-year PFS rate was lower in the SMO-mutant group (52%) than in the AKT1-mutant (83%) and “others” groups (82%) (P = .008).
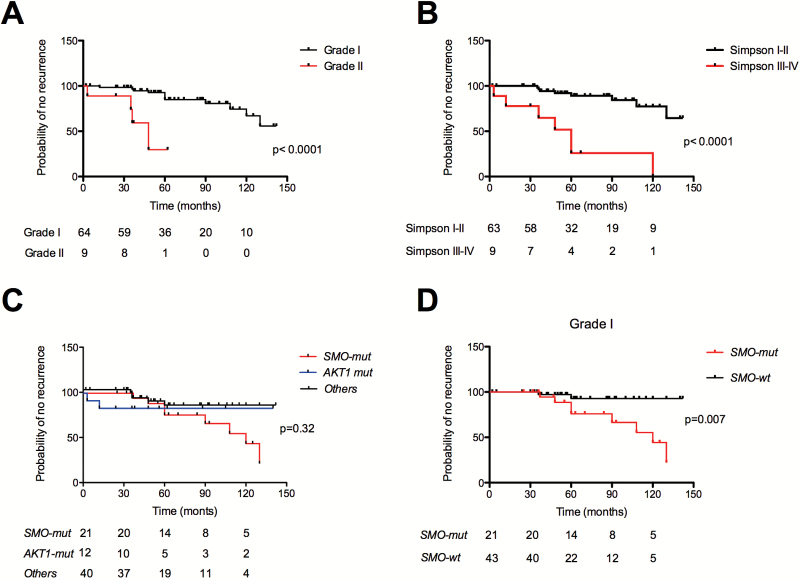
WHO grade II (P < .0001, Fig. 2A) and Simpson grade III or IV resection (P < .0001, Fig. 2B) were associated with poor survival, but no significant differences were observed according to the 3 molecular groups (P = .32) (Fig. 2C), or age (<70 vs ≥70 y) (P = .33). When focusing the analysis on grade I OGM (n = 62), we observed that the SMO-mutant group had a significantly poorer prognosis (P = .007) (Fig. 2D). On multivariate analysis, WHO histological grade II (P = .006) and incomplete resection (P = .001) were independently associated with shorter recurrence-free survival, whereas SMO-mutation did not reach statistical significance (P = .06).
Fig. 2.
Kaplan–Meier plots of time to recurrence, according to (A) WHO grade (grade I vs grade II, P < .0001); (B) extent of resection (Simpson I–II vs Simpson III–IV, P < .0001); (C) molecular subgroups for the whole cohort (SMO-mutant [SMO-mut], AKT1-mutant [AKT1-mut] and “others” meningiomas [P = .32, NS]), and (D) for grade I only: SMO-mutants have a poorer prognosis than SMO-wt (P = .008). wt = wildtype.
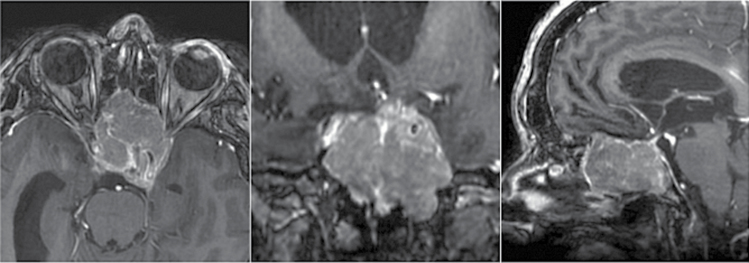
When reviewing the patterns of relapse of OGM, we identified 4 cases (5%) of surgery- and radiation-refractory meningiomas with nasal fossa and paranasal sinus invasion associated with severe visual impairment and pituitary dysfunction. Three of them carried targetable mutations (2 had SMOL412F, and 1 had the AKT1 mutation) (Fig. 3).
Fig. 3.
Axial (left), coronal (center), and sagittal (right) T1-weighted MRI scans with gadolinium enhancement showing invasive skull base recurrence of a SMO-mutant olfactory groove meningioma previously operated on 3 times and treated with radiotherapy in a 45-year-old woman. Both optic canals and pituitary gland and both carotid arteries are invaded and the patient suffers from pituitary dysfunction and blindness.
Discussion
We focused the analysis of this large OGM series on 2 frequent and potentially actionable mutations. Indeed, specific SMO and AKT1 inhibitors are under evaluation and will certainly provide new therapeutic options in the near future. This is not the case for NF2 mutations (rarely mutated in anterior skull base meningothelial meningiomas), KLF4 mutations (specific for secretory meningioma, absent in this cohort), and TRAF7 mutations. We identified a targetable mutation in almost half of tumors (43%). SMO (L412F or W535L) mutations were found in 28% of OGM, much more frequently than in meningiomas in any other location (3% to 5%),14,17,18 confirming its association with a specific topography and histology (grade I, meningothelial subtype). SMO is a 7-transmembrane domain protein belonging to the sonic hedgehog (SHH) pathway that plays a key role in embryogenesis and is implicated in several cellular processes, including proliferation, differentiation, and angiogenesis.23 The strong association of the SMO mutation with anterior skull base location is probably related to the central role of the SHH pathway in the development of the ventral forebrain and median craniofacial skeleton.24,25 Our results concerning histological characterization of SMO-mutant meningioma as meningothelial grade I lesions in all cases are in line with the findings summarized in a recent review of published cases of SMO-mutant meningiomas.20
AKT1 E17K was mutated in 15% of OGM, which is similar to the 12% found in non-NF2 meningiomas in any location.14,19 We confirmed that the olfactory groove is not particularly associated with the AKT1 mutation, in line with previous reports on AKT1-mutant meningiomas of the anterior clinoid process, tuberculum sellae, clivus, sphenoid wing, or spine.14,17,18 If most AKT1-mutant meningiomas were grade I and meningothelial (67%), they showed a more heterogeneous profile with regard to histological subtype (atypical, chordoid) as published in the literature.19–21
Apart from WHO grade II and incomplete resections which were associated with shorter PFS as expected,7 we also observed that the SMO-mutant meningiomas had an overall recurrence rate higher than the AKT-mutant or “others” group. Moreover, all patients who experienced late recurrence (after 5 y) had a SMO-mutant meningioma, and these were exclusively grade I meningiomas. On multivariate analysis, the prognostic value of molecular characterization did not reach statistical significance, making further studies with a large cohort mandatory to confirm our results. Yuzawa and colleagues recently reported a 6% recurrence rate for SMO-mutant meningiomas, but data were only available for 8 patients, and length of FU was not mentioned.20,21 As the WHO 2016 classifications introduced the use of integrated histological and genotypic parameters to define more focused histomolecular diagnostic entities, such molecular information should also help guide and improve the current classification and therapeutic management of meningiomas. The prognostic value of AKT1 mutations in OGM is less clear, and our results in this subgroup must be taken with caution because of the small number of cases: 4 patients with grade II and 2 patients with incomplete resections at recurrence. Additional studies with larger cohorts and other anatomical topographies are needed to assess the specific prognostic value of AKT1 mutations in skull base meningiomas.
Based on our results, we recommend a simple molecular analysis of OGM (with at least SMOL412F and AKT1E17K mutation status) after initial surgery to define more precisely the prognosis and extend FU for SMO-mutant meningiomas. In our OGM series, we have identified at least 3 cases that had unfavorable outcomes and for whom efficient targeted medical therapies would have provided an invaluable therapeutic tool. Large series of skull base meningiomas with precise location information and at least SMO and AKT1 mutation analysis and long-term FU with PFS analysis (as we have performed for OGM) are needed to establish new treatment paradigms and FU decisions based on genetic classification as in other brain tumors.26
Funding
This work was supported by a grant from the Fondation ARC (PJA 20131200431), France. J.B. was funded by a grant from UM1 University, Montpellier, France.
Conflict of interest statement. The authors declare no competing financial interests.
Acknowledgments
We are indebted to Marine Giry and Amithys Rahimian for technical assistance and Shai Rosenberg for statistical analyses.
References
- 1. Louis DN, Ohgaki H, Wiestler OD. WHO Classification of Tumours of the Central Nervous System. Revised 4th ed Lyon: International Agency for Research on Cancer; 2016. [Google Scholar]
- 2. Nakamura M, Struck M, Roser F, et al. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007;60(5):844–852. [DOI] [PubMed] [Google Scholar]
- 3. Obeid F, Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery. 2003;53(3):534–542; discussion 542–543. [DOI] [PubMed] [Google Scholar]
- 4. Pallini R, Fernandez E, Lauretti L, et al. Olfactory groove meningioma: report of 99 cases surgically treated at the Catholic University School of Medicine, Rome. World Neurosurg. 2015;83(2):219–231-3. [DOI] [PubMed] [Google Scholar]
- 5. Bassiouni H, Asgari S, Stolke D. Olfactory groove meningiomas: functional outcome in a series treated microsurgically. Acta Neurochir (Wien). 2007;149(2):109–121; discussion 121. [DOI] [PubMed] [Google Scholar]
- 6. Domingues PH, Sousa P, Otero Á, et al. Proposal for a new risk stratification classification for meningioma based on patient age, WHO tumor grade, size, localization, and karyotype. Neuro-Oncol. 2014;16(5):735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomasello F, Angileri FF, Grasso G, et al. Giant olfactory groove meningiomas: extent of frontal lobes damage and long-term outcome after the pterional approach. World Neurosurg. 2011;76(3-4):311–317; discussion 255–258. [DOI] [PubMed] [Google Scholar]
- 9. Gande A, Kano H, Bowden G, et al. Gamma knife radiosurgery of olfactory groove meningiomas provides a method to preserve subjective olfactory function. J Neurooncol. 2014;116(3):577–583. [DOI] [PubMed] [Google Scholar]
- 10. Kaley T, Barani I, Chamberlain M, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro-Oncol. 2014;16(6):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruttledge MH, Sarrazin J, Rangaratnam S, et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994;6(2):180–184. [DOI] [PubMed] [Google Scholar]
- 12. Bi WL, Abedalthagafi M, Horowitz P, et al. Genomic landscape of intracranial meningiomas. J Neurosurg. 2016;125(3):525–535. [DOI] [PubMed] [Google Scholar]
- 13. Domingues P, González-Tablas M, Otero Á, et al. Genetic/molecular alterations of meningiomas and the signaling pathways targeted. Oncotarget. 2015;6(13):10671–10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kros J, de Greve K, van Tilborg A, et al. NF2 status of meningiomas is associated with tumour localization and histology. J Pathol. 2001;194(3):367–372. [DOI] [PubMed] [Google Scholar]
- 16. Clark VE, Harmancı AS, Bai H, et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet. August 2016;48(10):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abedalthagafi M, Bi WL, Aizer AA, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro-Oncol. January 2016;18(5):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sahm F, Bissel J, Koelsche C, et al. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathol (Berl). 2013;126(5):757–762. [DOI] [PubMed] [Google Scholar]
- 20. Yuzawa S, Nishihara H, Tanaka S. Genetic landscape of meningioma. Brain Tumor Pathol. 2016;33(4):237–247. [DOI] [PubMed] [Google Scholar]
- 21. Yuzawa S, Nishihara H, Yamaguchi S, et al. Clinical impact of targeted amplicon sequencing for meningioma as a practical clinical-sequencing system. Mod Pathol Off J U S Can Acad Pathol Inc. 2016;29(7):708–716. [DOI] [PubMed] [Google Scholar]
- 22. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng JMY, Curran T. The Hedgehog’s tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11(7):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeong J, Mao J, Tenzen T, et al. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18(8):937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xavier GM, Seppala M, Barrell W, et al. Hedgehog receptor function during craniofacial development. Dev Biol. 2016;415(2):198–215. [DOI] [PubMed] [Google Scholar]
- 26. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]