Figure 2.
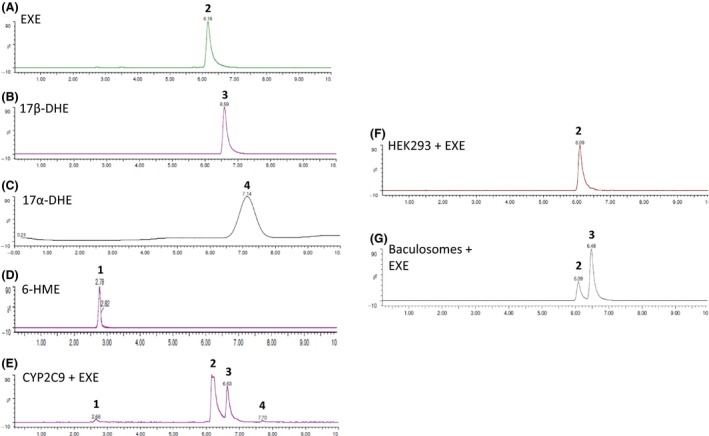
Identification of EXE metabolites. Panel (A), EXE (6‐methyleneandrosta‐1,4‐diene‐3,17‐dione) standard; panel (B), 17β‐DHE (17β‐hydroxy‐6‐methyleneandrosta‐1,4‐dien‐3‐one) standard; panel (C), 17α‐DHE (17α‐hydroxy‐6‐methyleneandrosta‐1,4‐dien‐3‐one) standard; panel (D), 6‐HME (6‐hydroxymethylandrosta‐1,4,6‐triene‐3,17‐dione) standard; panel (E), EXE metabolite profile after incubating CYP2C9‐overexpressing HEK293 microsomes with EXE; panel (F), EXE metabolite profile after incubating microsomes from the parent HEK293 cell line (no CYP450 overexpression) with EXE; panel (G), EXE metabolite profile after incubating negative control commercial baculosomes (no CYP450 overexpression) with EXE. Incubations were performed for 45 min at 37°C with 100 μM exemestane and 20 μg of CYP450‐overexpressing HEK293 microsomes, 50 μg of non‐CYP450‐overexpressing HEK293 microsomes or 50 μg of baculosomes. Peak 1, 6‐HME; peak 2, exemestane; peak 3, 17β‐DHE; peak 4, 17α‐DHE.
