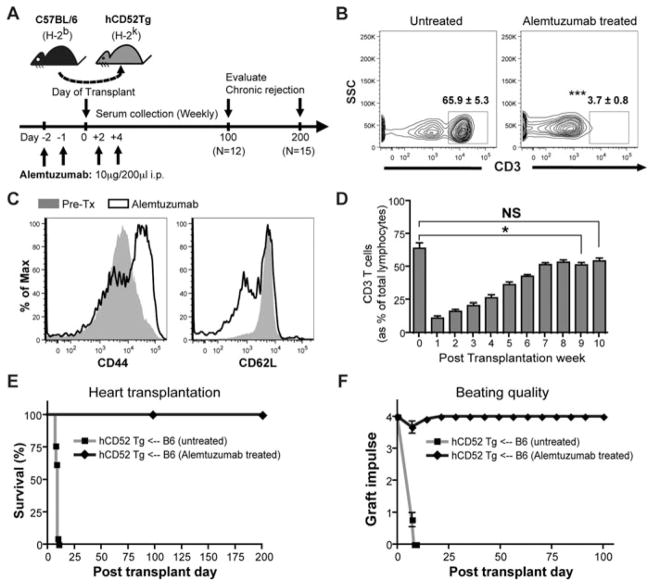
Figure 1. Alemtuzumab treatment induces prolonged full MHC mismatched cardiac allograft survival with profound T cell depletion in CD52Tg murine recipients.
(A) Alemtuzumab dosing strategy and experiment are represented. C57BL/6 (H-2b) hearts were transplanted into the CD52Tg mice (H-2k). Recipients were treated with total four doses of 10 μg of alemtuzumab. (B) Representative dot plots of T cells in the peripheral blood before and after alemtuzumab treatment. Flow cytometric measurements demonstrated profound depletion of CD3 T cells on day 0 (in the gate) in the peripheral blood. The numbers depict the average percent (±SEM). n = 4 – 5 mice per group. (C) Phenotypic analysis of T cells during repopulation. Repopulating T cells showed phenotypic switching from CD44loCD62Lhi (grey area) to CD44hiCD62lo (solid line) population. (D) T cell repopulation kinetics after alemtuzumab treatment with cardiac allograft. Serial frequencies of repopulating T cells were expressed as percent of T cells (CD3+) and of total lymphocytes (CD45+). n = 5 – 7 mice per time point. (E) Cardiac allograft survival was prolonged with alemtuzuamab treatment (MST > 100 days, n = 12 and MST > 200 days, n = 15) while untreated recipients showed acute rejection at MST of 8.0 days (n = 12). (F) Posttransplant graft function measured by abdominal palpation (grade 0–4). Strong 3–4+ graft beating was maintained in alemtuzumab treated recipients until day 200, whereas beating quality dropped to less than 2+ within 7 days after transplantation and stop completely in untreated recipients. n = 12 per group. *p < 0.05, ***p < 0.001, NS > 0.05.