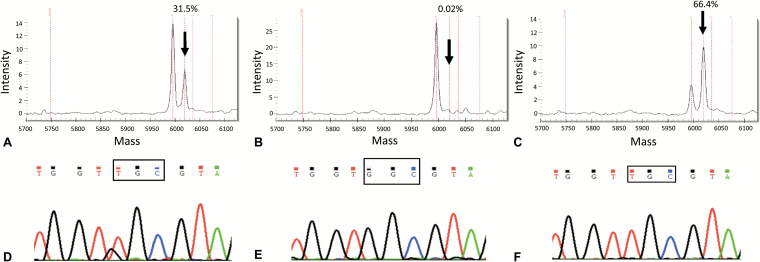
Fig. 1.
Detection of DNA alterations in the tumor tissue and in liquid biopsies of Case 1. The KRAS mutation c.37G>T, pG13C was diagnosed by Sequenom (Myriapod Lung Cancer kit, Diatech Pharmacogenetics) in the tumor resection sample (A); the estimated mutant allele frequency was 30% (arrow), suggesting a homozygous KRAS mutation, since the tumor cell content in the selected tumor area accounted for about 30%. The same driver mutation was not detected in plasma cfDNA samples (B), but was identified in CSF (C). The mutant allele frequency of >50% found in the CSF sample (arrow) suggested a likely homozygous pattern, in a way akin to the primary tumor. The highly sensitive fast COLD-PCR assay detected the same mutation in the tissue sample (D), confirming also the negativity of plasma (E) and positivity of CSF (F), using DNA input comprised in the range of absolute assay reproducibility.3