Abstract
Background.
Adaptive immune resistance in the tumor microenvironment appears to attenuate the immunotherapeutic targeting of glioblastoma (GBM). In this study, we identified a tumor-infiltrating myeloid cell (TIM) population that expands in response to dendritic cell (DC) vaccine treatment. The aim of this study was to understand how this programmed death ligand 1 (PD-L1)–expressing population restricts activation and tumor-cytolytic function of vaccine-induced tumor-infiltrating lymphocytes (TILs).
Methods.
To test this hypothesis in our in vivo preclinical model, we treated mice bearing intracranial gliomas with DC vaccination ± murine anti–PD-1 monoclonal antibody (mAb) blockade or a colony stimulating factor 1 receptor inhibitor (CSF-1Ri) (PLX3397) and measured overall survival. We then harvested and characterized the PD-L1+ TIM population and its role in TIL activation and tumor cytolysis in vitro.
Results.
Our data indicated that the majority of PD-L1 expression in the GBM environment is contributed by TIMs rather than by tumor cells themselves. While PD-1 blockade partially reversed the TIL dysfunction, targeting TIMs directly with CSF-1Ri altered TIM expression of key chemotactic factors associated with promoting increased TIL infiltration after vaccination. Neither PD-1 mAb nor CSF-1Ri had a demonstrable therapeutic benefit alone, but when combined with DC vaccination, a significant survival benefit was observed. When the tripartite regimen was given (DC vaccine, PD-1 mAb, PLX3397), long-term survival was noted together with an increase in the number of TILs and TIL activation.
Conclusion.
Together, these studies elucidate the role that TIMs play in mediating adaptive immune resistance in the GBM microenvironment and provide evidence that they can be manipulated pharmacologically with agents that are clinically available. Development of immune resistance in response to active vaccination in GBM can be reversed with dual administration of CSF-1Ri and PD-1 mAb.
Keywords: cancer, checkpoint inhibitors, CSF-1R, dendritic cell vaccine, glioblastoma, immunotherapy, PD-1
Importance of the study
Previous studies have suggested that tumor cells themselves are the relevant source of PD-L1 in the tumor microenvironment. Our work herein changes this paradigm, clearly demonstrating that the meaningful contribution of PD-L1 to the glioblastoma tumor microenvironment is instead from TIMs and that experimental manipulation of TIMs with PD-1 mAb treatment and CSF-1R inhibition can dramatically alter the progression of tumors and overall survival in animal models. It is these cells that actively suppress antitumor T-cell responses, and this fact must be accounted for in order to create more effective immunotherapies. Currently, there are 2 PD-1 mAbs approved for clinical use; and PLX3397, the CSF-1R inhibitor we utilized in these studies, is currently in phase III clinical trials. The findings presented herein are directly applicable to ongoing and upcoming clinical investigations and can be immediately applied to our vaccine-treated GBM patient population.
Glioblastoma (GBM) is one of the most lethal of human cancers, with very few long-term survivors and no definitive cures for this disease. Current therapies for GBM are largely palliative, and novel treatment strategies, such as immunotherapy, suggest promise.1–4 Active vaccination strategies have shown enough promise against GBM1,5 that a randomized, phase III clinical trial utilizing dendritic cell (DC) therapy in patients with new diagnoses is currently under way. In previous studies, however, extended survival associated with DC vaccination was variable, with evidence suggesting that persistent residual or progressive disease may impair the beneficial antitumor response.1,5
Our recent findings, and those of others, suggest that antitumor immune responses induced by active vaccination may be subsequently accompanied by an adaptive immune resistance mechanism mediated by signaling of programmed death 1 and its ligand (PD-1/PD-L1).6–8 PD-1/PD-L1 signaling has been documented in GBM,9–11 and early studies proposed that tumor cells themselves are the dominant expressers of PD-L1.7,12,13 However, PD-L1 is also known to be expressed by various cell populations of myeloid lineage that are frequently heterogeneously present in the tumor microenvironment and not necessarily by the tumor cells.14–20
Tumor-infiltrating myeloid cell (TIM) populations have been evaluated in a variety of tumor models. The absence of these cells appears to lead to markedly depressed tumor growth rates,21,22 and the infiltration of tumor-associated macrophages appears to increase in response to treatments such as radiation,23 chemotherapy,24 and immunotherapies,25 and thereafter promote tumor progression.26–30 These treatments induce colony stimulating factor 1 (CSF-1) secretion from tumors, which promotes the influx of myeloid cells into tumors. Once there, they increase expression of T-cell inhibitory factors, such as prostaglandin-E2, transforming growth factor–β, and interleukin (IL)-10, and promote tumor progression.23,24,31–33 Although TIMs analyses have been done in gliomas,34–36 the mechanisms by which this cell population influences antitumor immunity is largely unknown.
In this study, we identified that functional PD-L1 is mainly expressed by an expanded GBM TIM population following vaccination. We showed that PD-1 monoclonal antibody (mAb) blockade diminished the immunoregulatory effects of TIMs on the activation of tumor-specific T cells. We also showed that while treatment with colony stimulating factor 1 receptor inhibitor (CSF-1Ri) in our vaccinated GBM model did not fully deplete TIMs or abolish PD-L1 expression on TIMs, it did promote increased cytokine and chemokine signaling by TIMs, and appeared to support an increased tumor-infiltrating lymphocyte (TIL) influx in both ex vivo human GBM and in vivo murine glioma studies. Combined adjuvant PD-1 mAb and CSF-1Ri treatment together with vaccination significantly enhanced antitumoral immune responses in a murine glioma model and our validation in ex vivo GBM patient samples. Further, in our murine glioma model, these vaccine adjuvants together promoted significantly increased survival over treatment with either adjuvant alone.
Materials and Methods
Cell Lines and Human Specimens
All murine and human glioma tumor cells were cultured using complete Dulbecco’s modified Eagle’s medium (Mediatech) with supplementary 10% fetal bovine serum (FBS; Gemini Bio-Products), and 1% (v/v) penicillin and streptomycin (Mediatech Cellgro). Cells were then maintained in a humidified atmosphere of 5% CO2 at 37°C conditions. The Brain Tumor Translational Resource (BTTR) at UCLA provided paraffinized human GBM tissue pre– and post–DC vaccine treatment. Fresh tumor samples were obtained from patients with newly diagnosed GBM immediately following resection and digested in collagenase for 24 hours. These patients provided informed consent and were participating under our phases I and II DC vaccine clinical trials (NCT00068510 and NCT01204684). Patient-derived biological samples were procured under a UCLA Brain Tumor Bank protocol that was approved by the institutional review board. Discontinuous Percoll gradient separation was used to isolate distinct leukocyte populations from tumors. For fresh tumor, patients provided informed consent for their tissue to be used for research purposes, which was approved by the UCLA Medical Institutional Review Board. Additional details are provided in the Supplementary material.
Murine Model
GL261 glioma cells (2 × 104 in 2 μL PBS) were stereotactically injected intracranially into female C57BL.6 mice (see the Supplementary material). Following intracranial tumor implantation, mice were randomized into treatment groups (n = 6–12/group). Mice were obtained from the Division of Experimental Radiation Oncology at the University of California Los Angeles and housed in a defined flora and pathogen free vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Animal treatment was compliant with the University of California Los Angeles animal care policy and approved by the Chancellor’s Animal Research Committee.
Generation of Bone Marrow–Derived DC, Pulsing, and Vaccination
GL261 glioma cells were harvested and exposed to 3–5 freeze-thaw cycles. Lysate concentration was quantified using a Bradford protein assay (Bio-Rad). DCs were prepared from murine bone marrow progenitor cells and pulsed with 250 μg/mL GL261 lysate for 18 hours prior to treatment. DCs (1 × 106 cells/mouse) were then administered subcutaneously at 4 sites on the dorsal aspect of the mouse midbody on days 3 and 13 following tumor implantation.
In vivo Treatments and Depletions
Anti–PD-1 mAb (RMP1-14, BioXCell) was administered i.p. for 3 days a week at 250 mg/kg/day. Ly6-C (Monts 1, BioXCell) and CD8 (Lyt 2.1, BioXcell) depleting antibodies were administered i.p. at 200 mg/kg every other day. The CSF-1Ri (PLX3397, Plexxikon) was administered by oral gavage at 50 mg/kg/day.
Tumor Tissue Harvests and Flow Cytometry
Mouse tumor-bearing brain hemispheres were harvested 72 hours following the second DC vaccine treatment and prepared for flow cytometry and immunohistochemistry (IHC) as previously described.37 Fluorochrome conjugated antibodies to mouse CD3, CD4, CD8, CD25, Ly6-C, GR-1, CD45.2, CD11b, CD11c, F4/80, CSF-1R, Thy1.2, PD-1, and PD-L1 were obtained from eBioscience. World Health Organization grades III and IV gliomas were obtained from consenting patients shortly after surgical resection. A minimum of 2 g tissue was obtained for this study. A minimum of 1 × 106 TILs were isolated from tissue as previously described.37 Fluorochrome conjugated antibodies to human CD3, CD8, and CD11b were obtained from eBioscience as well. Flow cytometry was performed on an LSRII (BD Biosciences), and cell sorting was performed with a FACSAria (BD Biosciences). Gates were set based on fluorescence minus one (FMO). Data were analyzed using FlowJo (Treestar) software. Sorted lymphocytes were placed into culture using Roswell Park Memorial Institute (RPMI) medium supplemented with 10% FBS and 1% (v/v) penicillin and streptomycin. Additional details are provided in the Supplementary material.
Immunohistochemistry
Murine tissue was stained via IHC methods with the assistance of the UCLA Translational Pathology Core Laboratory for CD8 (4SM15, 1:100, eBioscience) and CD11b (M1/70, 1:100, eBioscience). Human tissue was stained via multiplex immunofluorescent methods at our facility using CD8, CD163, PD-1, PD-L1, and glial fibrillary acidic protein. Additional details are provided in the Supplementary material. Analysis for all tissue—including cell mapping, automated total cell count (total 4′,6′-diamidino-2-phenylindole positive [DAPI+]), and cell population counts (fluorochrome-positive cells and fluorochrome colocalization)—was performed using the Vectra 3.0 (PerkinElmer) quantitative pathology imaging system and inForm (PerkinElmer) analysis software.
TIL:TIM Transwell Assay
Thy1.2− CD11b+ TIMs and Thy1.2+ CD3+ TILs were subjected to fluorescence activated cell sorting (FACS) from the tumor-bearing hemispheres of DC vaccinated mice. TIMs were cultured in 24-well plates at 100000 cells/well in RPMI medium supplemented with 10% FBS, 1% (v/v) penicillin and streptomycin, and 100 IU/mL IL-2. TILs were added to 0.40 µm pore polycarbonate membrane transwell inserts at TIL:TIM ratios of 1:1 and 10:1. Recombinant murine interferon-gamma (IFNɣ) (Peprotech) was added to media at 100 or 1000 IU/mL, and a neutralizing IFNɣ mAb (XMG1.2, BioXCell) was added to media at 50 µg/mL. All cultures were for 24 hours before performing cellular analysis.
Quantitative Transcriptional Profiling
FACS was performed on tumor-infiltrating leukocytes from murine brain tumor–bearing hemispheres for Thy1.2− CD11b+ TIM and Thy1.2+ CD3+ TIL. RNA was obtained using an RNeasy mini kit (Qiagen) and analyzed using the nCounter GX Nanostring Analysis system (Nanostring Technologies), which allows for exact quantification of RNA expression via direct binding to tagged probes sampling approximately 770 genes.38,39 Data were then analyzed using the Nanostring nSolver Analysis software (Nanostring Technologies).
xCELLigence Real-Time Cytotoxicity Assay
For human ex vivo cytotoxicity studies, CD3+ TILs and CD11b+ TIMs from surgically resected patient gliomas were digested in collagenase and DNAse, enriched on a discontinuous Percoll gradient (30%–70%), subjected to FACS, and placed into culture with patient GBM (CD3− CD11b−) cells. Because of variation in overall cell count across samples, tumor cell:TIL:TIM ratio per co-culture was maintained at 1:10:1 with a minimum total tumor cell count of 5 × 104 and maximum of 1 × 105 cells per culture. For murine in vitro cytotoxicity studies, Pmel-1 gp100-specific T cells were placed into culture with GL261-gp100 glioma cells and FACS-subjected CD11b+ TIMs from tumor-bearing brain hemispheres of vaccinated mice. For murine studies, the tumor cell:TIL:TIM ratio was 1:10:1, with a tumor cell count maintained at 1 × 105 cells. Where indicated, T-cell media was supplemented with 10 μM anti–PD-1 antibody (BioXCell) for 1 hour on ice prior to the addition of T cells to xCELLigence assay. Where indicated, TIMs were cultured with 10 µM CSF-1Ri. Tumor cytolysis over 10 hours following co-culture was assessed using the xCELLigence Real-Time Cell Analyzer System (Acea Biotechnology).40,41 Afterward, cells from cultures containing tumor cells or tumor cells + TIMs were harvested and stained for PD-L1 expression. Tumor cells were identified as CD11b− cells.
In vitro Activation of Pmel-1 T Cells
Spleens and lymph nodes were harvested from Pmel-1 T-cell receptor transgenic mice. The organs were processed and a single-cell suspension was obtained and then cultured with human IL-2 (100 IU/mL, NCI Preclinical Repository, Developmental Therapeutics Program) and hgp100 (25–33) peptide (1 µg/mL, NH2-KVPRNQDWL-OH, Biosynthesis) in XVIVO-15 (Lonza) and 2% FBS. After 72 hours, cells were washed with PBS 1× and cultured in fresh media with 100 IU/mL IL-2 for an additional 3 days prior to use in cytotoxicity assays.
CRISPR Knock-out
Clustered regularly interspaced short palindromic repeat knock-out (CRISPR KO) of PD-L1 in the GL261-gp100 cell line was performed as described previously.42 Additional details are provided in the Supplementary material. Transduced cells were purified using puromycin selection followed by FACS of PD-L1(−) cells from the puromycin-resistant population.
Results
GBM-Infiltrating TIMs Inhibit T-Cell Mediated Tumor Cytolysis via the PD-1/PD-L1 Axis
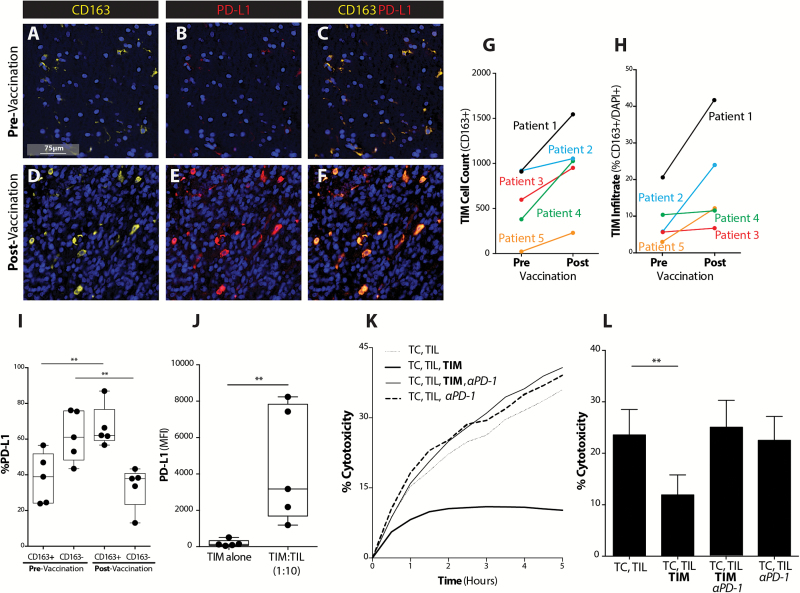
Our group has previously shown that the PD-1/PD-L1 negative costimulatory system reduces the efficacy of vaccination-induced immune responses in established intracranial tumors. To understand the mechanisms underlying these cellular interactions, we examined pre- and post-vaccinated patient GBM tumor samples using multiplex IHC. Surprisingly, we found that the majority of PD-L1 expression in the tumor microenvironment was present on CD163+ cells of myelomonocytic lineage, both pre- and post-vaccination (Fig. 1A–F) (Supplementary Fig. 1). Interestingly, not only was there an increase in the number of TIMs expressing PD-L1 following vaccination (Fig. 1G), but the density of TIMs in vaccinated established tumors became greater (Fig. 1H). TIMs were the dominant contributors of PD-L1 in the tumor microenvironment, especially after DC vaccination (Fig. 1I).
Fig. 1.
GBM TIMs expand to inhibit vaccine-induced T-cell mediated tumor cytolysis via PD-1/PD-L1 regulatory pathway. (A, D) CD163, DAPI, (B, E) PD-L1, DAPI, and (C, F) CD163, PD-L1, and DAPI co-staining are shown across pre- and post-DC vaccination samples from a GBM patient at 40× magnification (scale bar represents 75µm). (G) CD163+ cell count across pre– and post–DC vaccine treatment patient samples was quantified (n = 5). (H) Percent of CD163+ cells of total number of cells (DAPI+) was quantified (n = 5). (I) The percent of CD163+ cells dually expressing PD-L1 was quantified before and after vaccination (n = 5) (**P < .01). (J) PD-L1 expression on CD11b+ TIMs in the absence or presence of CD3+ TILs from freshly resected GBM shown (**P < .01) (n = 5). (K) Representative plot demonstrating TIL cytolysis of tumor cells (TC) over time in the absence or presence of TIMs or PD-1 mAb shown for freshly resected GBM. (L) GBM tumor cell cytolysis at selected time point of 4 hours in the absence or presence of TIMs or PD-1 mAb shown (n = 11/group) (**P < .01).
In order to interrogate the immunoregulatory effects of TIMs, tumor cells and TILs were cultured with or without TIMs from freshly resected GBM patient tumor specimens. In the presence of TILs, TIMs significantly upregulated PD-L1 expression (Fig. 1J). Lysis of patient-derived primary tumor cells by the autologous TILs was significantly reduced in the presence of TIMs, but addition of a PD-1–blocking mAb to this TIM-TIL-tumor cell co-culture recovered the lysis to levels identical to cultures without TIMs (Fig. 1K, L). Interestingly, in the absence of TIMs, PD-1 blockade did not provide any additional TIL cytolytic benefit, suggesting that the functional PD-1/PD-L1 interaction was predominantly between TIMs and TILs (Fig. 1l).
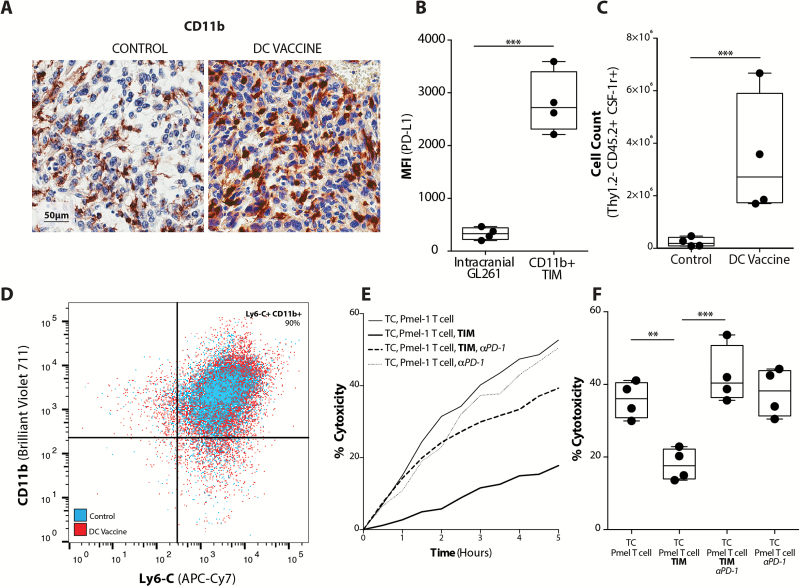
In the murine GL261 glioma model, we identified an analogous CD11b+ myelomonocytic population that similarly expanded after DC vaccination in large, established tumors (Fig. 2A). These murine TIMs demonstrated significantly greater PD-L1 expression compared with glioma cells (Fig. 2B, Supplementary Fig 2). This TIM population was further characterized by multicolor FACS as non–T-lymphocyte (Thy1.2−), monocyte lineage (CD11b+ and Ly6-C+), Gr-1−, CSF-1R+, and F4/80+ cells (Fig. 2C, D) (Supplementary Fig. 3). The Ly6-C and F4/80 expression on these cells was not affected by the DC vaccination treatment (Supplementary Fig. 4). To evaluate whether the expression of PD-L1 by TIM also suppressed cytotoxic T-cell activity in the murine model, we co-cultured CD11b+ cells sorted by FACS from intracranial tumors with GL261-hgp100 glioma cells and Pmel-1 hgp100-specific T cells. As seen with human GBM, the presence of TIMs significantly reduced the T-cell cytolytic ability (Fig. 2E, F) and this could be recovered with PD-1 mAb treatment ex vivo, but PD-1 blockade did not have any apparent effect on tumor cell lysis in the absence of TIMs. To confirm that PD-L1 expression by tumor cells did not functionally suppress T-cell mediated tumor cytolysis, we created GL261-hgp100 glioma cells in which the PD-L1 gene was disrupted. Lysis of control, PD-L1–sufficient GL261 cells was not different when compared with PD-L1–deficient CRISPR GL261-hgp100 cells, regardless of PD-1 blockade in vitro (Supplementary Fig. 5). These data suggested that PD-L1–mediated immune suppression was not primarily mediated by tumor cells. Rather, TIMs functionally used this pathway to inhibit T-cell induced tumor cytolysis in the glioma microenvironment.
Fig. 2.
Murine glioma TIMs expand in response to vaccination to inhibit T cell-mediated tumor cytolysis. (A) CD11b IHC staining of nontreatment control and DC vaccinated tumor-bearing mice (scale bar represents 50µm). (B) Mean fluorescence intensity for PD-L1 expression on FACS intracranial GL261 gliomas (CD11b− CD3− cells) and TIMs (CD11b+ CD3−); (n = 4/group) (***P < .001). Flow cytometric characterization of (C) the absolute number of Thy1.2−, CD45.2+, CSF-1R+ cells (n = 4/group) (***P < .001), and (D) representative scatter plot of percent CD11b+, Ly6-C+ TIMs from tumors of control and DC vaccinated mice. (E) Representative xCELLigence plot of Pmel-1 T cell cytolysis of GL261-hgp100 cells (TC) over time in the absence or presence of TIMs or PD-1 mAb. (F) Quantitation of Pmel-1 T-cell induced tumor cell cytolysis at 4 hours in the absence or presence of TIMs or PD-1 mAb (n = 4) (**P < .01, ***P < .001).
The Recruitment of TIMs that Express High Levels of PD-L1 Is an Adaptive Immune Response to Vaccine-Induced TILs in the Glioma Microenvironment
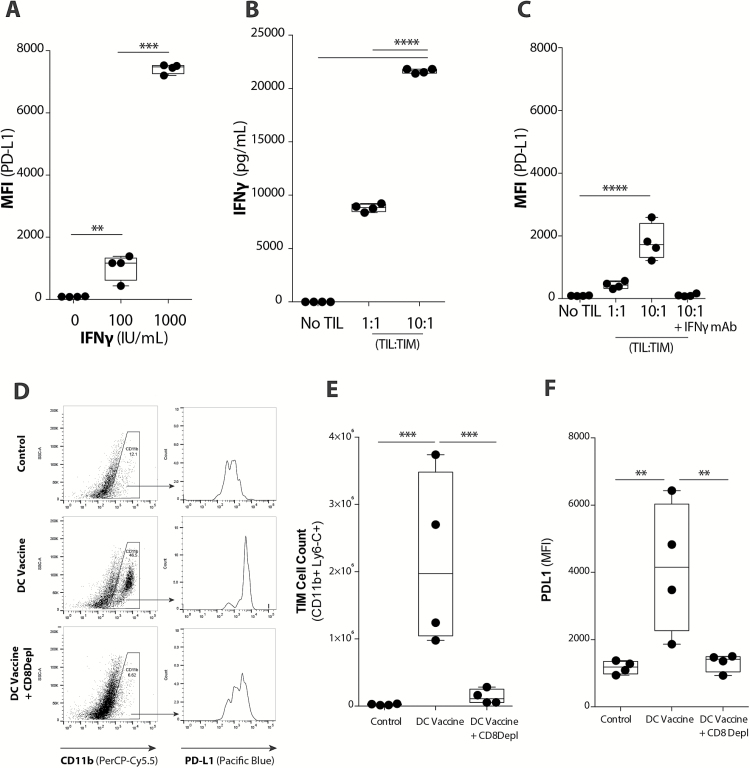
These previous results suggested that the antitumor immune response induced by DC vaccination also elicited a compensatory recruitment of TIMs. As such, we hypothesized that TILs might affect the phenotype of GL261 glioma-infiltrating TIMs. The addition of IFNɣ to GL261 tumor cell cultures did increase PD-L1 expression on those cells, but T-cell lysis of tumor cells in vitro was not affected (Supplementary Fig. 6). However, the addition of increasing concentrations of IFNɣ to TIMs cultured ex vivo resulted in dramatic increases in PD-L1 expression on TIMs that was proportionally more significant than the PD-L1 upregulation on tumor cells (Fig. 3A). Supernatants from TIM-TIL transwell co-cultures were analyzed using a multi-analyte cytokine assay, which demonstrated significantly increased IFNɣ levels with increasing numbers of TILs (Fig. 3B). As a result of such TIL:TIM co-cultures, there was also a proportional increase in the expression of PD-L1 when TILs were increased (Fig. 3C). Neutralization of IFNɣ in these TIM-TIL co-cultures completely abolished this TIL-related PD-L1 upregulation on TIMs (Fig. 3C). In vivo, post-vaccination TIM expansion (Fig. 3D, E) and PD-L1 upregulation on these TIMs (Fig. 3F) were significantly reduced when CD8+ T cells were depleted in vaccinated mice. These results suggest that TIM upregulation of PD-L1 was an adaptive resistance mechanism elicited by the antitumor activity of vaccine-induced IFNɣ-secreting CD8+ TILs.
Fig. 3.
TIMs upregulate PD-L1 in response to vaccine-induced TIL population. (A) Upregulation of PD-L1 on TIMs in the presence of increasing IFNɣ concentrations was quantified with flow cytometry (n = 4/group) (***P < .001). (B) IFNɣ levels present in TIL-TIM co-cultures in vitro was quantified using a Luminex assay (n = 4/group) (****P < .0001). (C) Upregulation of PD-L1 on TIMs in the presence of increasing concentrations of TILs or blockade of IFNɣ (IFNɣ mAb) was quantified with flow cytometry (n = 4/group) (****P < .0001). (D) Representative plots demonstrate PD-L1 expression on the CD11b+ TIM population across treatment groups. (E) CD11b+ Ly6-C+ TIM cell count and (F) PD-L1 expression in non-treatment control, DC vaccinated, and DC vaccinated + CD8 mAb depletion tumor-bearing mice (n = 4/group) (**P < .01, ***P < .001). Statistical analyses were performed using Student’s t-test (A–F).
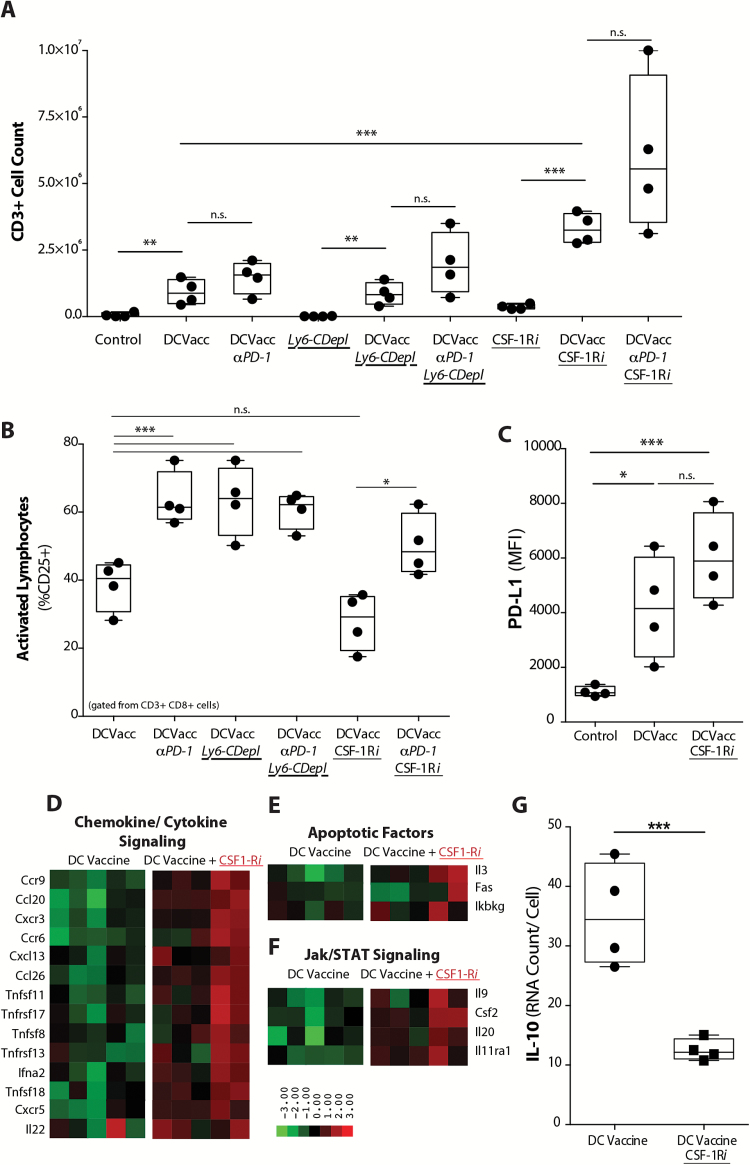
We next evaluated the corresponding influence of TIMs on TILs. Tumor-bearing mice treated with DC vaccine, PD-1 blockade, or a Ly6-C mAb that depletes TIMs (Supplementary Fig. 7) were assessed for TIL infiltration and activation. Although TIM depletion alone did not promote an infiltrating T-cell response, when it was combined with DC vaccination there was a significantly increased number of TILs found (Fig. 4A). Activation markers in the CD8+ TIL were also significantly elevated, comparable to levels observed with PD-1 mAb blockade (Fig. 4B). However, PD-1 mAb blockade in addition to DC vaccine with Ly6-C antibody depletion in tumor-bearing mice did not provide any additional increase in T-cell activation compared with animals treated with DC vaccine and Ly6-C depletion alone. These results demonstrated that TIMs mediated T-cell suppression and that blockade of PD-1 was functionally similar to depletion of TIMs in vivo.
Fig. 4.
TIM-mediated PD-1/PD-L1 immunoregulatory mechanism reduces T cell infiltration and activation in tumor. (A) Absolute TIL count (CD3+) and (B) TIL activation (%CD8+ CD3+ CD25+) in tumors from mice treated with DC vaccine and PD-1 mAb, along with Ly6-C depleting mAb (Ly6-C Depl) or CSF-1Ri (n = 4/ group) (*P < .05, **P < .01, ***P < .001, ****P < .0001). (C) PD-L1 expression across treatment groups (n = 5/group) (**P < .01, ***P < .001). Unbiased heatmap ranking and quantification of (D) chemokine/cytokine signaling, (E) apoptotic factors, and (F) Jak/STAT pathway signaling factors on CD11b+ TIMs from tumor-bearing mice receiving DC vaccination alone or with adjuvant CSF-1Ri treatment (n = 5/group). (G) Quantification of IL-10 expression by CD11b+ TIMs from tumor-bearing mice treated with DC vaccine or DC vaccine + CSF-1Ri is shown. (n = 4/group) (***P < .001).
PD-1 mAb and CSF-1Ri Together Maximally Enhance the Vaccine-Generated Immune Response
Since the in vivo depletion of myeloid cells is not feasible clinically, we evaluated whether a small molecule inhibitor of CSF-1R was effective in our model. For our studies, we utilized a small molecule CSF-1R inhibitor (PLX3397, Plexxikon). While CSF-1Ri treatment in unvaccinated mice did not significantly alter the infiltrating T-cell response, we showed that CSF-1R blockade in vaccinated glioma-bearing mice led to reduced infiltrating TIM populations (Supplementary Fig. 7) and significantly increased TIL infiltration (Fig. 4A). When compared with the earlier experiments with DC vaccine + Ly6-C TIM depletion, these effects were more profound. Although DC vaccine + CSF-1Ri increased overall TIL infiltration above that induced by DC vaccine treatment alone, it did not significantly alter TIL activation (Fig. 4B). This led us to hypothesize that CSF-1Ri–treated TIMs affected TILs through mechanisms other than PD-L1, as TIMs continued to express stable levels of PD-L1 after treatment with CSF-1Ri (Fig. 4C).
To further understand the increase in the CD3+ TIL population seen with adjuvant CSF-1Ri treatment, we used a quantitative transcriptional profiling assay to evaluate the gene expression profile of TIMs from mice treated with DC vaccination or DC vaccine + CSF-1Ri. An elevation in chemotactic, apoptotic, and Janus kinase/signal transducer and activator of transcription (Jak/STAT) signaling factors following CSF-1Ri treatment was observed, which could potentially explain the increased TIL infiltration findings (Fig. 4D–F). Interestingly, IL-10 was significantly downregulated and other known M1/M2 markers also showed decreased expression with CSF-1Ri treatment (Fig. 4G) (Supplementary Fig. 8). While other immuno-inhibitory factors are also expressed by this population of cells (eg, transforming growth factor–β, IL-6) and could potentially contribute to the suppressive milieu of these tumors, our data strongly suggest that PD-L1 and IL-10 play dominant roles in our model. These results suggest that CSF-1Ri treatment altered the gene expression profile of intratumoral TIM to that which favored the recruitment of T lymphocytes.
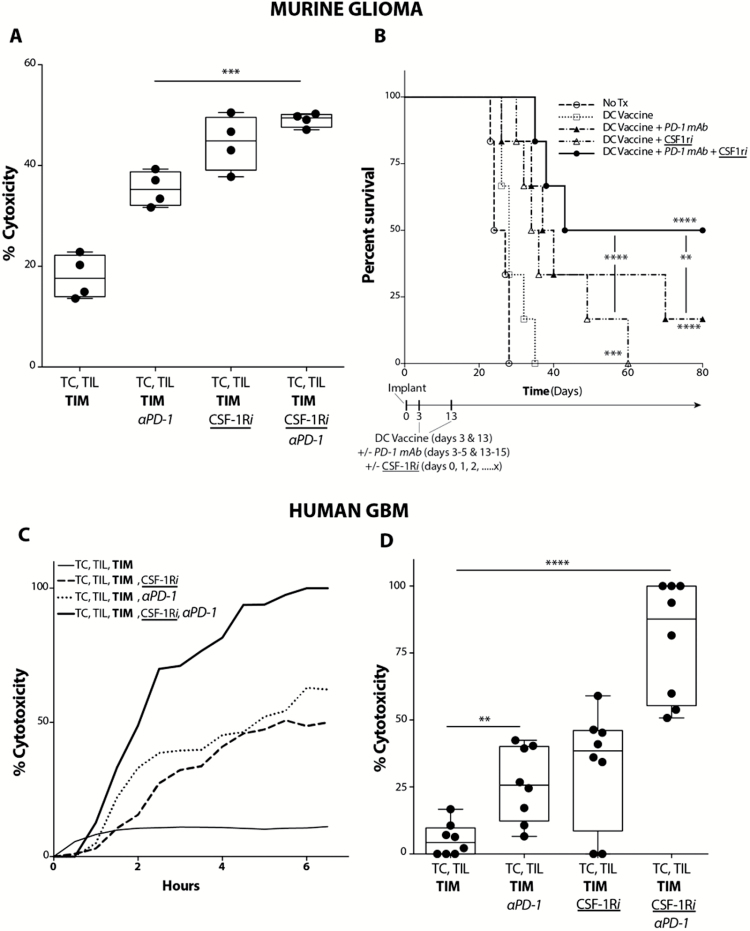
Based on our findings, CSF-1Ri and PD-1 blockade appeared to influence 2 independent aspects of the antitumor immune response. CSF-1Ri promoted an increased density of the TIL population generated by the DC vaccine treatment, and PD-1 blockade activated the TIL population. When combined, these 2 adjuvants were able to synergistically enhance the DC vaccine treatment. With murine cells, there was a significant increase in ex vivo tumor cytolysis with combination therapy compared to treatment with PD-1 blockade alone (Fig. 5A). In vivo, DC vaccine with PD-1 blockade significantly increased survival over DC vaccination alone, as did DC vaccine with CSF-1Ri (Fig. 5B). However, DC vaccine with CSF-1Ri and PD-1 blockade together significantly increased long-term survival of tumor-bearing mice compared with mice that received DC vaccination with either treatment alone. Importantly, similar effects were observed in our ex vivo GBM patient tumor samples. When patient GBM-derived TILs were co-cultured with autologous patient GBM TIMs and tumor cells, TIL-mediated tumor cytolysis was enhanced with either PD-1 blockade or CSF-1Ri alone versus nontreated controls. However, we saw a further significant increase in TIL-mediated tumor cytolysis with PD-1 mAb and CSF-1Ri combined compared with either treatment alone (Fig. 5C, D).
Fig. 5.
Combination treatment with PD-1 mAb and CSF-1Ri maximally enhances vaccination-induced immune responses in both murine glioma and ex vivo human GBM. (A) Murine GL261-hgp100 glioma cell (TC) cytolysis at 4 hours following co-culture of TC, Pmel-1 T cells, and TIMs FACS-subjected from intracranial tumor-bearing mice treated with DC vaccination. PD-1 mAb or CSF-1Ri were added to Pmel-1 T cells or TIM ex vivo, respectively (n = 4/ group) (***P < .001). (B) Mice were randomized into control (tumor-bearing, no treatment), DC vaccine, DC vaccine + PD-1 mAb, DC vaccine + CSF-1Ri, and DC vaccine + PD-1 mAb + CSF-1Ri treatment groups. Graph depicts comparison of survival using method of Kaplan–Meier (n = 6/group) (**P < .01, ***P < .001, ****P < .0001) (P values indicate statistical difference from no treatment control unless otherwise indicated). (C) Human GBM tumor cell (TC) cytolysis over time following co-culture of TC, TIL, and TIM for representative GBM patient. PD-1 mAb or CSF-1Ri were added to autologous TIL or TIM ex vivo, respectively. (D) Compilation of human GBM tumor cell (TC) cytolysis at 4 hours following co-culture of TC, TIL, and TIM. PD-1 mAb or CSF-1Ri were added to TIL and TIM ex vivo, respectively (n = 8 patient samples/group) (**P < .01, ****P < .0001).
Discussion
We have previously established that vaccination is necessary to generate an infiltrating immune response in a non-immunogenic cancer such as GBM and that the interaction of PD-1–expressing TILs with PD-L1 in the tumor microenvironment reduces its efficacy.6 Our initial hypothesis was that tumor cells mediated this suppression of TILs by a PD-1/PD-L1 dependent mechanism as a compensatory reaction to restrict the vaccine-generated immune response. However, our data from both patient GBM samples and murine preclinical models strongly indicate that TIMs are responsible for the functional PD-L1 expression within the tumor microenvironment. Our in vitro data initially suggested that both TIMs and tumor cells upregulated PD-L1 in response to IFNɣ. However, tumor cell expression of PD-L1 in both intracranial murine brain gliomas and patient-derived GBM cells was insignificant compared with PD-L1 expression by TIMs. The expression of PD-L1 by a nontumor cell population, as opposed to the tumor cells themselves, within the tumor microenvironment is an important distinction. This distinction suggests that functional reprogramming of TIMs, instead of tumor cells, may be more important for enhancing vaccine approaches to GBM. It will likely prove necessary to overcome the local inhibitory effects of this cell population to sustain a successful antitumor immune response.
We were able to isolate endogenous infiltrating populations of TILs in patients with newly diagnosed GBM without prior treatment. When these TILs were placed into co-culture with GBM tumor cells at a sufficient effector-to-target ratio, there was significant tumor cytolysis, confirming that these TILs have the capacity to exert a functional tumor-specific response. Given that these tumors progress despite the accumulation of tumor-specific TIL populations, such TILs are clearly not able to mediate significant enough tumor cytolysis to halt tumor progression. In our clinical and preclinical studies, DC vaccination appears capable of boosting the numbers of glioma-infiltrating TILs. However, we found that there is a reciprocal TIM expansion associated with this treatment. In response to TIL-secreted factors, TIMs expand and inhibit TIL activation and tumor cytolysis directly via the PD-1/PD-L1 signaling pathway. The expression of PD-L1 on such TIM populations suggests that this negative costimulatory pathway may dominantly mediate adaptive immune resistance in glioma.
Our studies using the CSF-1R inhibitor PLX3397 gave us further insight that TIMs have multiple roles in the tumor microenvironment. It should be noted that while “tumor-associated macrophage” is a commonly used term for the myeloid cells we call TIMs, our evidence suggests that “macrophage” is too restrictive a term. The glioma-infiltrating myeloid populations include dendritic cells and other myeloid cells in various stages of differentiation within the tumor. Although CSF-1R inhibition did not fully deplete the TIM population or alter TIM PD-L1 expression, it did alter the gene expression signature of these cells. TIMs from mice treated with DC vaccine and CSF-1Ri showed elevated chemokine, cytokine, and Jak/STAT transcripts. The sum total effect of these pathways may be to enhance TIL recruitment and expansion. Future therapies might be utilized to transform these inhibitory cell populations to support a continued antitumor immune response.
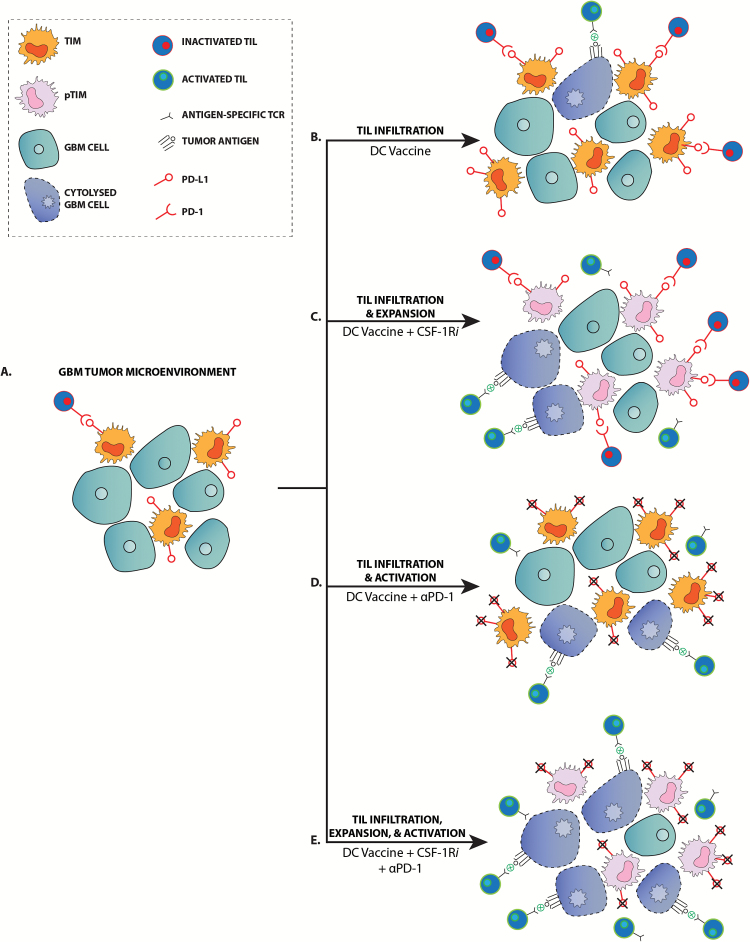
Interestingly, PD-1 mAb blockade or CSF-1Ri treatment together with DC vaccination promote significantly increased survival. The evidence suggests that each treatment targets a different aspect of the antitumor immune response (Fig. 6). When PD-1 mAb blockade is used in conjunction with DC vaccination, we observed increased activation of TILs. When we used a CSF-1R inhibitor in conjunction with DC vaccination, we saw an expansion of the TIL population and an altered pro-inflammatory TIM (pTIM) population. Independently, each adjuvant treatment enhances one aspect of the DC vaccine–induced immune response. Together, however, these 2 treatments may both activate and expand the TIL population such that there is significant increase in tumor cytolysis and survival.
Fig. 6.
PD-1 mAb blockade and CSF-1R inhibitors enhance vaccine-induced immune responses by distinct mechanisms. (A) Malignant gliomas lack a significant antitumor immune response in the nontreatment setting. (B) DC vaccination elicits an infiltrating TIL response, but is largely ineffective due to inactivation of TILs via the PD-1/PD-L1 signaling mechanism. (C) Treatment with CSF-1Ri alters the TIM population to become pro-inflammatory TIM (pTIM) and results in the expansion of the TIL population, increasing the potential for TIL-tumor cell interaction and tumor cytolysis. (D) While PD-1 mAb treatment does not increase the TIL population over what is generated by DC vaccination, it does promote activation of TILs and subsequent tumor cytolysis. (E) PD-1 mAb and CSF-1Ri together promote the expansion and activation of the DC vaccine-generated TIL population such that there is a maximal tumor cytolysis.
The translational relevance of these adjuvant treatments was confirmed with both elevated tumor cytolysis in ex vivo patient GBM cultures and significantly prolonged survival in our preclinical murine glioma model. Classically M2-associated genes were downregulated in TIMs from CSF-1Ri–treated mice, in large agreement with other recent studies.43,44 However, to our knowledge, this study represents the first time that both PD-1 mAb and CSF-1Ri have been used together to enhance the active vaccination strategy in an immune-competent murine glioma model. Currently, there are 2 PD-1 mAbs approved for clinical use,45–47 and PLX3397, the CSF-1R inhibitor we utilized in these studies, is currently in phase III clinical trials. As such, we consider the findings in this study to be directly applicable to clinical investigations and propose that such adjuvant treatments be directly applied to our vaccine-treated GBM patient population. In non-immunogenic cancers that clearly need an active vaccination strategy to generate an immune response, such as glioma, this combination treatment may provide an exciting avenue for therapy by both enhancing and activating immunity in the tumor microenvironment.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported in part by NIH/NCI grants R21-CA186004 and R01CA154256 (R.M.P.), RO1 CA125244 (L.M.L.), R25 NS079198 (R.M.P., R.G.E., and L.M.L.), the Isabel Neidorf Foundation (R.M.P., L.M.L.), the Musella Foundation for Brain Tumor Research (R.M.P.), the UCLA Graduate Division Dissertation Year Fellowship (J.P.A.), and the UCLA Medical Scientist Training Program (M.S.T.P.) (J.P.A.).
Conflict of interest statement. None to declare.
Supplementary Material
Acknowledgments
We would like to thank the Center for Systems Biomedicine (Integrated Molecular Technologies Core) for their technical assistance with the Nanostring studies, which is supported by CURE/P30DK41301-26. We also thank the UCLA Jonsson Comprehensive Cancer Center (JCCC) and the Center for AIDS Research Flow Cytometry Core Facility, which is supported by the National Institutes of Health awards P30 CA016042 and 5P30 AI028697. Finally, we would also like to thank the UCLA Brain Tumor Translational Resource (BTTR) and the Translational Pathology Core Laboratory (TPCL) for their assistance with paraffin-embedding and histology.
References
- 1. Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. [DOI] [PubMed] [Google Scholar]
- 2. Prins RM, Wang X, Soto H, et al. Comparison of glioma-associated antigen peptide-loaded versus autologous tumor lysate-loaded dendritic cell vaccination in malignant glioma patients. J Immunother. 2013;36(2):152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heimberger AB, Hussain SF, Aldape K, et al. Tumor-specific peptide vaccination in newly-diagnosed patients with GBM. J Clin Oncol. 2006;24(18):107s. [Google Scholar]
- 4. Kanaly CW, Ding D, Heimberger AB, et al. Clinical applications of a peptide-based vaccine for glioblastoma. Neurosurg Clin N Am. 2010;21(1):95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prins RM, Soto H, Konkankit V, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17(6):1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antonios JP, Soto H, Everson RG, et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2016;1(10): pii: e87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bloch O, Crane CA, Kaur R, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abiko K, Matsumura N, Hamanishi J, et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. [DOI] [PubMed] [Google Scholar]
- 14. Schultheis AM, Scheel AH, Ozretic L, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer. 2015;51(3):421–426. [DOI] [PubMed] [Google Scholar]
- 15. Qu QX, Huang Q, Shen Y, et al. The increase of circulating PD-L1-expressing CD68(+) macrophage in ovarian cancer. Tumour Biol. 2016;37(4):5031–5037. [DOI] [PubMed] [Google Scholar]
- 16. Krempski J, Karyampudi L, Behrens MD, et al. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol. 2011;186(12):6905–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soares KC, Rucki AA, Wu AA, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prall F, Huhns M. PD-L1 expression in tumour buds of colorectal carcinoma. Histopathology. 2016;69(1):158–160. [DOI] [PubMed] [Google Scholar]
- 20. Mathios D, Ruzevick J, Jackson CM, et al. PD-1, PD-L1, PD-L2 expression in the chordoma microenvironment. J Neurooncol. 2015;121(2):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin EY, Nguyen AV, Russell RG, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193(6):727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. [DOI] [PubMed] [Google Scholar]
- 23. Escamilla J, Schokrpur S, Liu C, et al. CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Res. 2015;75(6):950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73(9):2782–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stafford JH, Hirai T, Deng L, et al. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18(6):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Movahedi K, Guilliams M, Van den Bossche J, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233–4244. [DOI] [PubMed] [Google Scholar]
- 28. Gabrilovich D, Nefedova Y. ROR1C regulates differentiation of myeloid-derived suppressor cells. Cancer Cell. 2015;28(2):147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–421. [DOI] [PubMed] [Google Scholar]
- 30. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16(1):38–52. [DOI] [PubMed] [Google Scholar]
- 32. Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. [DOI] [PubMed] [Google Scholar]
- 33. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. [DOI] [PubMed] [Google Scholar]
- 34. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. [DOI] [PubMed] [Google Scholar]
- 35. Nishie A, Ono M, Shono T, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5(5):1107–1113. [PubMed] [Google Scholar]
- 36. Rossi ML, Jones NR, Candy E, et al. The mononuclear cell infiltrate compared with survival in high-grade astrocytomas. Acta Neuropathol. 1989;78(2):189–193. [DOI] [PubMed] [Google Scholar]
- 37. Antonios JP, Soto H, Everson R, et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2016;1(10). pii: e87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malkov VA, Serikawa KA, Balantac N, et al. Multiplexed measurements of gene signatures in different analytes using the Nanostring nCounter Assay System. BMC Res Notes. 2009;2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Payton JE, Grieselhuber NR, Chang LW, et al. High throughput digital quantification of mRNA abundance in primary human acute myeloid leukemia samples. J Clin Invest. 2009;119(6):1714–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peper JK, Schuster H, Loffler MW, et al. An impedance-based cytotoxicity assay for real-time and label-free assessment of T-cell-mediated killing of adherent cells. J Immunol Methods. 2014;405:192–198. [DOI] [PubMed] [Google Scholar]
- 41. Everson RG, Antonios JP, Lisiero DN, et al. Efficacy of systemic adoptive transfer immunotherapy targeting NY-ESO-1 for glioblastoma. Neuro Oncol. 2016;18(3):368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11(8):783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quail DF, Bowman RL, Akkari L, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352(6288):aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.