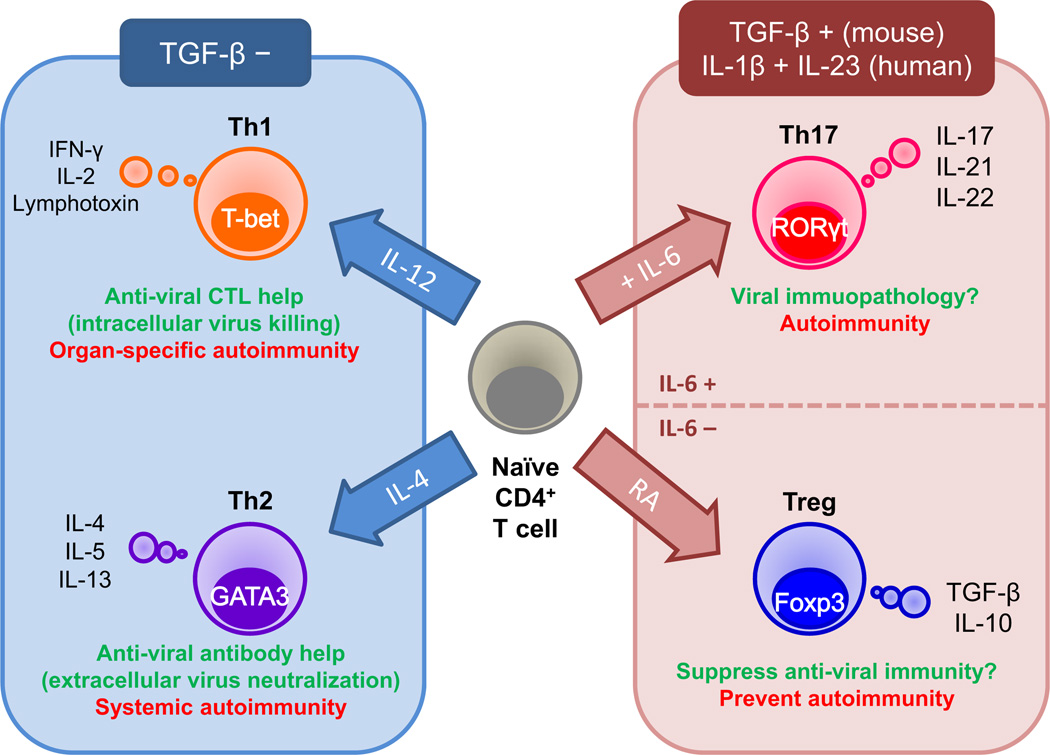
Figure 1.
Roles of T helper (Th) cell subsets in viral infections and autoimmunity. Th cell subset differentiation is influenced by the cytokine milieu and master transcription factor. Th1 cells are induced in the presence of interleukin (IL)-12 and the transcription factor, T-box expressed in T cells (T-bet). Th1 cells help induction of antiviral cytotoxic T lymphocytes (CTL) and activation of macrophages that eradicate intracellular virus, while Th1 cells can also be a pathogenic effector in organ-specific autoimmunity. Th2 cells are induced by IL-4 and the transcription factor, GATA-binding protein 3 (GATA3). Th2 cells help production of antiviral antibody that neutralizes extracellular virus, but can be detrimental to promote autoantibody production in systemic autoimmunity. Although Th1 and Th2 differentiation is inhibited by transforming growth factor (TGF)-β, both Th17 cells and regulatory T cells (Tregs) are induced by TGF-β. In addition, Th17 cell differentiation requires IL-6, whereas Treg differentiation requires retinoic acid (RA). Th17 cells express the transcription factor, retinoic acid receptor-related orphan receptor-γt (RORγt), secrete IL-17, 21 and 22, and might be involved in immunopathology in viral infections and autoimmunity. Tregs express the transcription factor, forkhead box P3 (Foxp3), and secrete the anti-inflammatory cytokines, TGF-β and IL-10, which can play a detrimental or protective role by suppressing antiviral immunity or autoimmunity, respectively.