Supplemental digital content is available in the text.
Abstract
Background
The affinity of IgG Fc receptor (FcγR) for rituximab, an anti-CD20 IgG1, differs based on single-nucleotide polymorphisms (SNPs) in FcγRs. This study aimed to explore the effect of such SNPs on clinical response to rituximab and outcomes in patients of ABO-incompatible (ABOi) living donor liver transplantation (LDLT).
Methods
SNPs of FCGR2A[131H/R] and FCGR3A[158F/V], alleles encoding FcγR, were identified in 20 patients desensitized with rituximab before ABOi LDLT. The effect of these SNPs on B cell elimination and outcomes was analyzed in the patients.
Results
The isoform encoded by FCGR2A[131H/H] had a higher affinity for IgG1, and accordingly, the effects of rituximab on B cells were more profound in individuals with FCGR2A[131H/H] than in individuals with FCGR2A[131H/R or R/R]. Specifically, the time to B-cell reappearance in the peripheral blood was significantly delayed, and total serum IgM levels were significantly lower early after LDLT in individuals with FCGR2A[131H/H], even though these SNPs did not significantly affect the reduction of antiblood group A/B antibodies. The incidence of blood stream infection was also significantly higher in individuals with FCGR2A[131H/H], and this SNP was associated with poor prognosis. Despite no significant effect of FCGR3A[158F/V] on survival after ABOi liver grafts, the incidence of infection was significantly higher in individuals with FCGR3A[158F/V or F/F] than in individuals with FCGR3A[158V/V].
Conclusions
Our findings indicate FCGR SNPs influence the effect of rituximab on B-cell depletion and are possibly predisposing factors for infectious complications after ABOi LDLT. This study will be a good foundation for further studies on larger cohorts.
To achieve successful ABO blood group-incompatible (ABOi) kidney transplantation, the prophylactic use of rituximab, a monoclonal chimeric human-murine anti-CD20 IgG1 that depletes B cells by complement-dependent cytotoxicity, antibody (Ab)-dependent cell-mediated cytotoxicity (ADCC), and stimulation of apoptosis,1,2 is currently indispensable.3 Treatment with rituximab has also been employed in adult ABOi living donor liver transplantation (LDLT), and it improved outcomes to levels close to those achieved in ABO-compatible LDLT,4 although the higher incidence of severe infectious complications in ABOi LDLT cases remains to be solved. Because ADCC is initiated by interactions between the Fc segment of IgG and Fc gamma receptors (FcγRs) on monocytes, macrophages, dendritic cells, and/or natural killer (NK) cells, allelic polymorphisms on genes encoding the FcγR on these immunocompetent cells potentially influence the effects of rituximab. One such polymorphism in the gene encoding FCGR3A [158F/V] (rs396991) is a single nucleotide substitution that leads to the replacement of phenylalanine (F) by valine (V) at amino acid position 158 in the IgG binding domain.5 IgG1 and IgG3 bind more strongly to FcγRIIIa [158V/V] than to [158F/F], thereby increasing effector cell activity in individuals with FCGR3A [158V/V].6-9 A single-nucleotide polymorphism (SNP) in the gene encoding FCGR2A [131H/R] (rs1801274) places either histidine (H) or arginine (R) at position 131.10 IgG1 binds more strongly to cells that have FcγRIIa [131H/H].6,9,11 These SNPs of FCGR2A and FCGR3A have been reported to be associated with the clinical outcome of rituximab in B cell non-Hodgkin lymphoma.12-14 Previous basic studies have also demonstrated the correlations between FCGR SNPs and the effect of ADCC, which are entirely consistent with the affinity of FcγR for IgG1, showing no opposing results.15,16 In contrast, some clinical analyses do not support a predictive role of FCGR2A and/or FCGR3A SNPs in the therapy of B cell malignancies with rituximab.17-19 Heterogeneous features of the B-cell malignancies, such as CD20 expression levels,20 HLA polymorphisms,21 and a variety of intrinsic apoptotic activity,18,22 or the combination of rituximab with other chemotherapy17 may help explain the inconsistent objective response rate in clinical analysis. Thus, numerous reports have described the association of FCGR2A and FCGR3A SNPs with the objective response rate of B cell malignancies to rituximab. However, none of the other analyses has shown the impact of SNPs on the effect of rituximab in ABOi organ transplantation. This is the first report on the influence of SNPs of FCGR2A and FCGR3A on the normal B-cell elimination, total serum immunoglobulin levels, and outcomes rituximab-based desensitization therapy for ABOi LDLT.
MATERIALS AND METHODS
Study Population and Immunosuppression Regimen
The cases (n = 20) that received ABOi LDLT under rituximab-based immunosuppressive regimen from April 2007 to March 2016 at the Hiroshima University were enrolled in this study. Life-threatening, severe comorbidities were not observed preoperatively in this cohort. Rituximab (375 mg/m2) was intravenously administered 2 weeks before the transplantation, whereas tacrolimus and mycophenolate mofetil were orally administered 2 weeks before the transplantation, and the target trough levels of tacrolimus were 5 to 10 ng/mL. Plasma exchange or double filtration plasmapheresis was performed to preserve the spleen in recipient of LDLT when high titer of the Ab was reduced. Simultaneous splenectomy had not been performed for immunosuppression. After transplantation, IVIg therapy was appropriately performed, which is required to maintain the total IgG levels in sera within the normal limit, without the aim of immunosuppression. This study was approved by the Institutional Review Board of Hiroshima University (No Hi-77, M625), and the study protocol conformed to the ethical guidelines of the Declaration of Helsinki, 1975. A written informed consent was obtained from all the patients.
Prophylactic Antibiotic Therapy
Antimicrobial prophylaxis consisted of intravenous cefmetazole (1.0 g/instance) (CEFMETAZON for intravenous injection; Daiichi Sankyo Company, Limited, Tokyo, Japan) administration shortly before surgery and every 6 hours during surgery; thereafter, a dosage of 2.0 g/d was maintained for 4 days.
Analysis of SNPs of FCGR2A [131H/R] and FCGR3A [158F/V]
Determination of SNPs of FCGR2A [131H/R] (rs1801274) and FCGR3A [158F/V] (rs396991) was done by restriction-fragment length polymorphism analysis. Genomic DNA was isolated from peripheral blood mononuclear cells or liver-derived mononuclear cells using Wizard SV Genomic DNA Purification System (Promega Corporation, Madison, WI), per manufacturer’s instructions. The genomic region containing the polymorphism was amplified by polymerase chain reaction, and the amplicon was purified using commercially available polymerase chain reaction product purification kit, KOD FX (TOYOBO, Fukui, Japan). Restriction-fragment length polymorphism analysis was done by enzymatic digestion with restriction enzymes, BstUI and N1aIII, (New England BioLabs, Ipswich, MA) for FCGR2A and FCGR3A, respectively. The enzymatically digested restriction fragments were separated on 2.5% agarose gels.
Residual B-Cell Analysis in Peripheral Blood and Spleen by Flow Cytometry
We performed spleen biopsy during ABOi LDLT desensitization with rituximab in all patients, except in patients who underwent splenectomy before transplantation. Our objective was to validate the elimination of B cells from the spleen and determine if the patient required splenectomy. The cells were stained with fluorescein isothiocyanate-conjugated anti-IgM (BD Pharmingen) and PE-conjugated anti-CD19 (BD Pharmingen) and analyzed using a fluorescence-activated cell sorter Calibur flow cytometer (Becton Dickinson). The rate of residual B cells in splenocytes and peripheral blood mononuclear cells was determined by selecting cells gated on IgM+ and CD19+ lymphocytes.
We performed splenectomy only if B cells were clearly detected in the spleen by flow cytometry analyses of the biopsy specimens at the time of transplantation (ie, in cases where more than 1% of IgM+ CD19+ cells were detected among the splenocytes).
Definition of Blood Stream Infection
Blood stream infections (BSI) accompanied by a bacteriological proof and clinical symptoms within 3 months after LDLT regardless of the presence or absence of other infectious diseases.
Statistical Analysis
The results were statistically analyzed using the Mann-Whitney U test. The Pearson χ2 test was used to analyze the incidence rate. Overall survival was calculated with the Kaplan-Meier method, and the statistical differences were determined by the log-rank test. Values with P less than 0.05 were considered as statistically significant.
RESULTS
Demographics and Characteristics of Patients
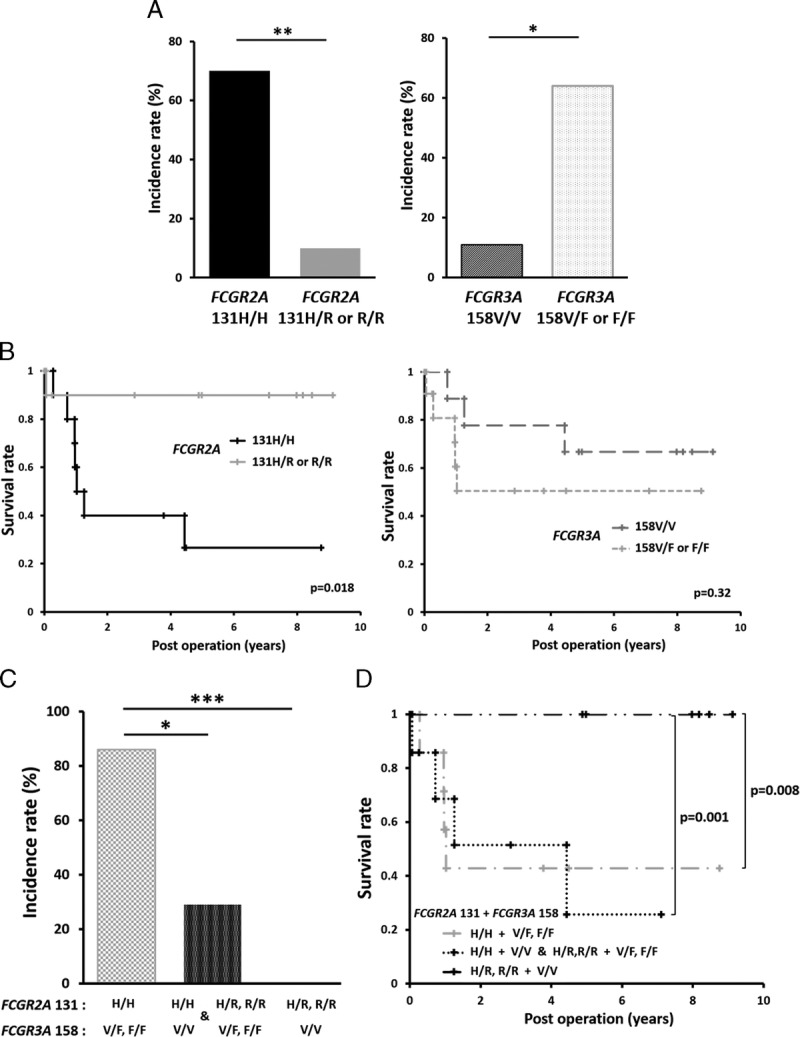
Genotyping data for FCGR2A [131H/R] and FCGR3A [158F/V] of 20 patients who received ABOi LDLT were obtained, which revealed that 10 patients were homozygous for FCGR2A [131H/H], the other 10 were FCGR2A [131R/R or R/H], whereas 9 patients were homozygous for FCGR3A [158V/V], 11 were FCGR3A [158F/F or F/V]. Comparative analyses of the characteristics of patients with FCGR2A and FCGR3A SNPs are listed in Table 1. The incidence of severe biliary complications was significantly higher in patients with FCGR2A [131H/H] than that in patients with FCGR2A [131R/R or R/H]. The number of FCGR2A [131R/R or R/H] patients treated with radiofrequency ablations for hepatocellular carcinoma (HCC) was significantly higher as compared with the FCGR2A [131H/H] patients. Further, the proportion of hepatitis B virus cases for FCGR3A [158F/F or F/V] patients was significantly lower as compared with that for the FCGR3A [158V/V] patients. However, these diseases did not seem to influence patient mortality. No other statistically significant differences in patient characteristics were observed. Neither elevation of antiblood group isoagglutinin titers nor Ab-mediated rejection was observed after transplantation in any of the patients, as previously reported.23
TABLE 1.
Characteristics of ABO-incompatible LDLT recipients with FCGR2A and FCGR3A polymorphism
No Differences Were Observed in the Proportion of Residual B cells in the Spleen Regardless of SNPs of FCGR2A and FCGR3A
The immunocompetent cells with FcγRIIa H-allele have been reported to have a remarkably higher affinity to human IgG1 than those with R-allele, raising the question of whether there is difference in effects of rituximab with respect to the FCGR2A SNPs.6,9,11 Similarly, in FcγRIIIa, immunocompetent cells with V-allele has a slightly higher affinity for IgG1 than those with F-allele,6-9 although the affinity of FcγRIIIa to IgG1, per se, is generally lower than that of FcγRIIa, regardless of genotypes.9 Despite such theoretical projection, flow cytometry analyses for biopsy specimens of the spleen collected at the time of transplantation revealed no differences in the proportion of residual B cells regardless of the SNPs of FCGR2A and FCGR3A (Figure S1, SDC, http://links.lww.com/TXD/A42).
Effects of Rituximab on B Cells Were More Profound in Individuals With FCGR2A [131H/H] Than in Individuals With FCGR2A [131H/R or R/R]
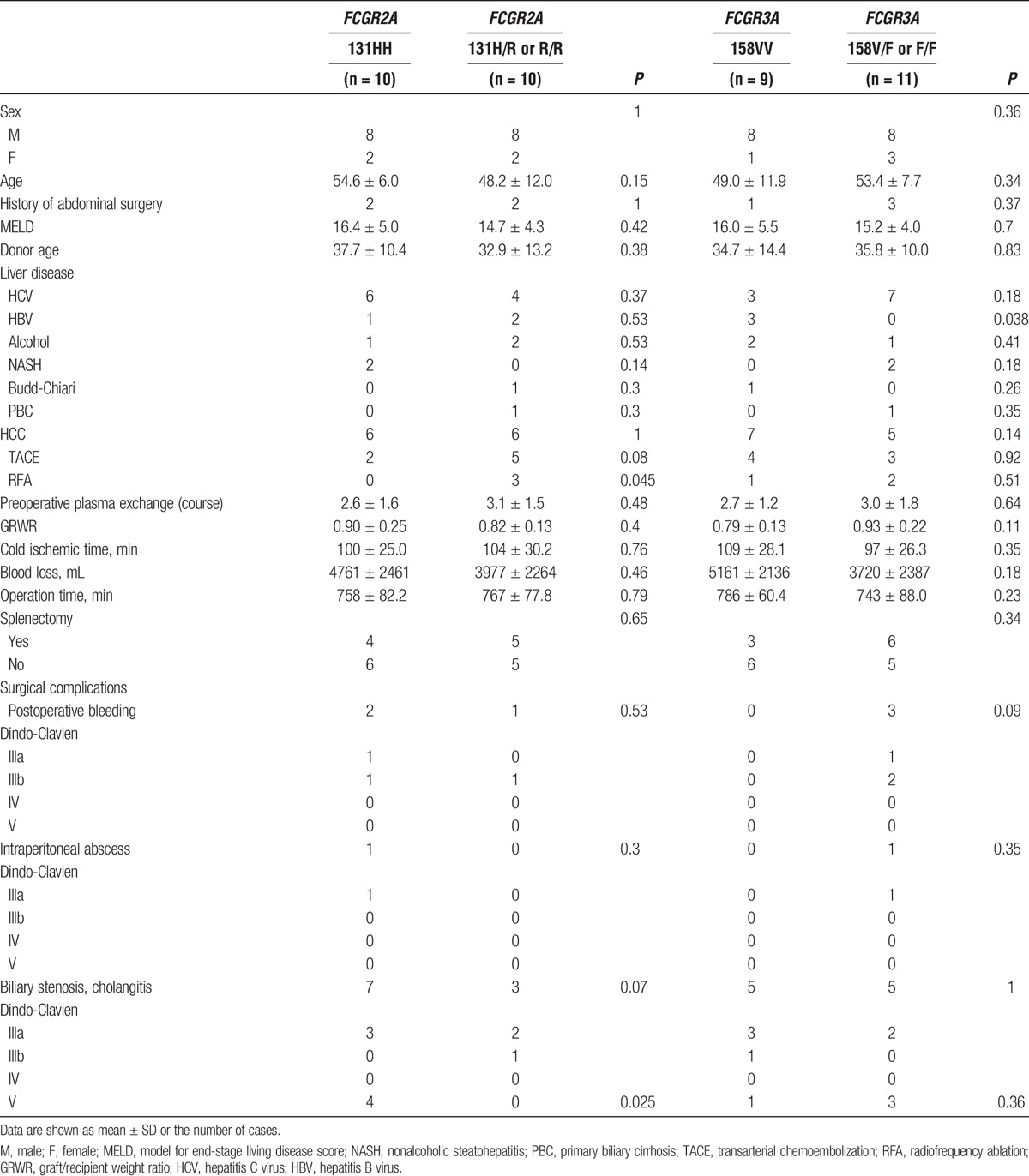
Regardless of the FcγR genotypes, B cells in the peripheral blood were unexceptionally abrogated at 1 week after the administration of a single dose of rituximab, and remained undetectable for 3 months after LT (Figure 1A). However, the time of B-cell reappearance in the peripheral blood was significantly delayed in individuals with FCGR2A [131H/H], consistent with a higher affinity of the isoform encoded by FCGR2A [131H/H] to IgG1 (Figure 1A). In addition, the total IgM levels in serum were significantly lower (beyond the lower limit of standard value) in the early period after LT in individuals with FCGR2A [131H/H] than in FCGR2A [131H/R or R/R] recipients (Figure 1B). Such a trend in serum IgM levels was not observed for the FCGR3A SNPs, probably reflecting the lower affinity of FcγRIIIa to IgG1 than that of FcγRIIa. Different from serum IgM levels, serum IgG levels were influenced to a similar level by administrating IVIg, which was generally indicated in patients with hypoglobulinemia. Hence, we analyzed both the serum IgG levels and total administered dose of IVIg in recipients of LT. The IgG levels were kept around lower limit of standard value by administrating IVIg during the early period after LT, with no difference with respect to SNPs of both FCGR2A and FCGR3A (Figure 1C). Noticeably, a significantly larger amount of IVIg was required in individuals with FCGR2A [131H/H] to maintain serum IgG levels comparable to those in individuals with FCGR2A [131H/R or R/R] (Figure 1D). This result suggests that net production of IgG might be further suppressed and/or existing IgG might be further consumed for immune defense against pathogens in individuals with FCGR2A [131H/H].
FIGURE 1.
Effects of rituximab on B cells were more profound in individuals with FCGR2A [131H/H] than in individuals with FCGR2A [131H/R or R/R]. A, A single dose of rituximab (375 mg/m2) was administered 2 weeks before transplantation. The proportion of IgM+CD19+ B cells in peripheral blood mono nuclear cells were determined by flow cytometry at different time points. Each point represents the mean and standard error of the mean at the individual time point as described. Representative gating strategy for the flow cytometry were shown. B, C, Total IgM (B) and IgG (C) levels in serum were analyzed at different time points as described. Dotted lines indicate lower limits of standard value. Each point represents the mean and standard error of the mean at the individual time point. D, Total dose of IVIg administered within 30 days after operation were shown. Data represent mean and standard error of the mean. The results were statistically analyzed using the Mann-Whitney U test. (FCGR2A [131H/H] : n = 10/[131R/R or R/H]: n = 10, FCGR3A [158V/V] : n = 9/[158F/F or F/V] : n = 11, *P < 0.05). RTX, rituximab.
FCGR SNPs Influence the Incidence of Infection and Outcomes of ABOi LDLT
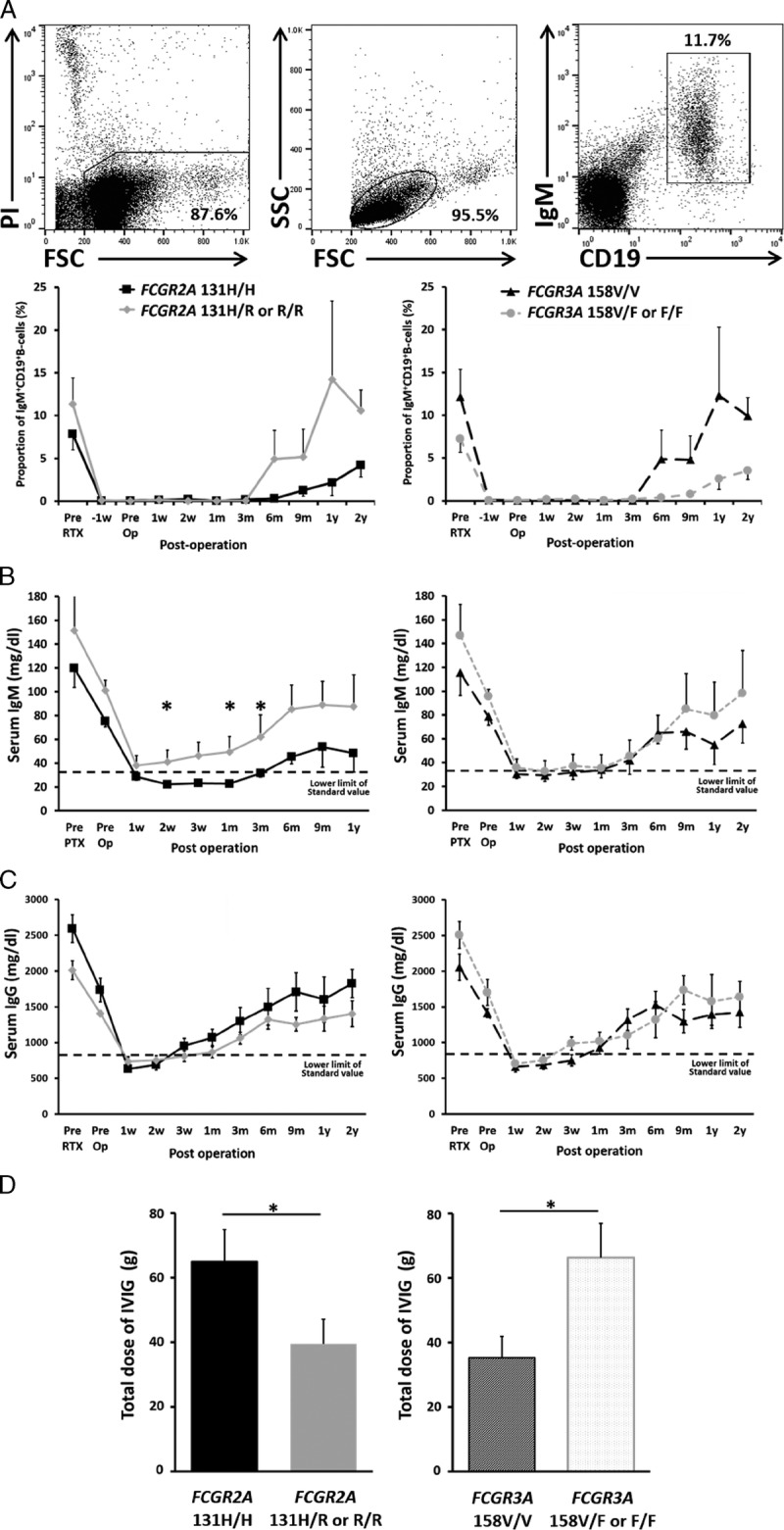
The severely suppressed immunoglobulin production in individuals with FCGR2A [131H/H] raised a question as to whether they are susceptible to infectious complications. Consistent with this assumption, the incidence of BSI was significantly higher in individuals with FCGR2A [131H/H] than that in individuals with FCGR2A [131H/R or R/R] (Figure 2A), and the prognosis of individuals with FCGR2A [131H/H] was markedly poor, whereas preferable outcomes were seen in individuals with FCGR2A [131H/R or R/R] (Figure 2B). With respect to FCGR3A SNPs, although the impact of the polymorphism on the prognosis was not remarkable (Figure 2B), significantly higher incidence of BSI was observed in individuals with FCGR3A [158F/F or F/V] than in individuals with FCGR3A [158V/V] (Figure 2A). We have recently demonstrated that the FCGR3A SNP in recipients of LT was greatly associated with the susceptibility to severe posttransplant infections, ie, a significantly higher incidence of BSI was observed in the individuals with FCGR3A [158F/V or F/F] than that in the individuals with FCGR3A [158V/V].24 This finding might reflect a fact that the affinity for IgG3, which induces vigorous compliment activation and ADCC, is remarkably higher in FcγRIIIa than that in FcγRIIa, and FCGR3A [158F/F or F/V] has much less affinity than FCGR3A [158V/V].9,24 Hence, FCGR3A SNPs likely influence innate components of humoral immunity.
FIGURE 2.
FCGR SNPs influence the incidence of infection and outcomes of ABOi LDLT. A, The incidence rate of BSIs within 3 months were shown. B, Postoperative survival rate were shown with the Kaplan-Meier method. (C) The incidence rate of BSIs within 3 months by the combination of FCGR2A and FCGR3A SNPs were shown. FCGR2A [131R/R or R/H] and FCGR3A [158V/V] is low risk combination (n = 6). FCGR2A [131H/H] and FCGR3A [158F/F or F/V] is high risk combination (n = 7). FCGR2A [131H/H] and FCGR3A [158V/V] or FCGR2A [131R/R or R/H] and FCGR3A [158F/F or F/V] are both high risk and low risk combinations (n = 7). *P < 0.05, ***P < 0.001. D, Postoperative survival rate by the combination of FCGR2A and FCGR3A SNPs were shown with the Kaplan-Meier method. The Pearson χ2 test was used to analyze the incidence rate, and the statistical differences of overall survival were determined by the log-rank test. Values with P < 0.05 were considered as statistically significant.
As FcγR classes differ in cell distribution and affinity for IgG subclasses, the combination of FCGR2A and FCGR3A SNPs possibly further stratifies the incidence of infectious complications. The low-risk combination, which consists of FCGR2A [131R/R or R/H] and FCGR3A [158V/V], showed excellent outcome: 8-year survival rate of 100%, whereas the other combinations (FCGR2A [131H/H] and FCGR3A [158F/F or F/V or V/V] or FCGR2A [131R/R or R/H] and FCGR3A [158F/F or F/V]) had significantly poor prognosis, indicating that SNPs of FCGR2A and FCGR3A influence the outcome of ABOi LDLT desensitized by rituximab (Figures 2C and D). Regarding cause of the death, 7 of the 8 death patients in the present study died of sepsis. These patients frequently sustained other complications, such as cholangitis, pneumonia, recurrence of hepatitis C virus, or acute rejection. All 7 patients suffered from BSI post-LT. Even though these patients successfully recovered from early phase BSI, they eventually died of sepsis. Only 1 patient died 5 years after LT because of HCC recurrence. These results suggest that a strong association exists between survival and infectious complications.
DISCUSSION
B-cell desensitization using rituximab has become an established strategy for ABOi LDLT. However, the higher incidence of severe infectious complications in ABOi LDLT cases than that in ABO-compatible LDLT cases remains to be resolved. The elimination of B cells sometimes caused hypoimmunoglobulinemia for both of IgG and IgM. A critical role of natural IgM in the immediate defense against several antigens has been well demonstrated,25 and previous studies suggested that polyvalent IgM-enriched human IVIg preparations are beneficial for obtaining favorable outcomes in sepsis.26 Because the IgM-enriched IVIg preparation is not available in Japan, polyclonal IgG could be solely supplemented with IVIg administration in the present treatment regimen. Hence, total IgM levels in sera are not influenced by administrating IVIg; in other words, the total IgM levels might directly reflect the few B cells remaining even after administration of rituximab. In this study, by evaluating the total IgM levels, we analyzed the effect of rituximab on the depletion of normal B cells with low variation in CD20 expression. This approach may have less bias than analyzing the clinical objective response rate of B cell malignancies with phenotypic variations to a therapy.18,20-22 Our results for binding affinities to IgG1 were consistent for FCGR2A SNP, but not for FCGR3A SNP. Since ADCC mediated through FcγR, carried by immunocompetent cells such as macrophages and NK cells, plays a critical role in the effect of IgG1 Ab therapy,27 FcγRIIa having higher affinity for IgG1 may have a stronger association with the effect of rituximab than with the lower affinity of FcγRIIIa for IgG1. Another reason for the remarkable association of the effect of rituximab with FCGR2A SNP but not with FCGR3A SNP might be the difference in cell distribution of FcγR classes, that is, FcγRIIa is expressed on neutrophils and macrophages, whereas FcγRIIIa is expressed on NK cells and macrophages.14 Considering the fact that the vast majority of immunocompetent cells bearing FcγRIIIa are NK cells15 and our previous finding that cytotoxic activity of NK cells is inhibited in patients suffering from severe liver failure who require liver transplantation,28 it is likely that ADCC-mediated B cell elimination through FcγR caused by rituximab administered before LDLT is dependent on the cytotoxicity of neutrophils and macrophages bearing FcγRIIa more than that of NK cells bearing FcγRIIIa.
Although B cells in the spleen at the time of transplantation were abrogated regardless of FCGR2A and FCGR3A SNPs, the time of B cell reappearance in the peripheral blood was significantly delayed and the total IgM levels in serum were significantly lower at the early period after LT in individuals with FCGR2A [131H/H]. This finding is consistent with the higher affinity of the isoform encoded by FCGR2A [131H/H] to IgG1 than that of the isoform encoded by FCGR2A [131H/R or R/R]. Taking into account a recent basic study using a mouse model, which demonstrated that all B cells in the spleen were depleted while those in the bone marrow remained detectable at the early period after injection of anti-CD20 monoclonal Ab,29 it is possible that the FCGR2A SNP is associated with the levels of remnant B cells in the bone marrow of LT patients treated with rituximab, thereby influencing the pace of B cell recovery in peripheral blood.
Immunoglobulin-directed activity plays a pivotal role in the innate immunity against microbes. Immunoglobulins with appropriate constant region isotypes (IgG1 and IgG3) can induce complement-dependent activities. In addition, they can recruit immunocytes and induce various activities through the interaction between IgG and FcγR on innate immune cells. This includes stimulation of bacterial phagocytosis by macrophages, induction of ADCC by macrophages or NK cells, and the generation of an oxidative burst from neutrophils. Further, IgM can also induce complement-dependent activities. Hence, the levels of IgG and/or IgM might play a key role in postoperative infectious complications. Since the isoform encoded by FCGR3A [158V] has greater affinity to both IgG1 and IgG3 than those encoded by FCGR3A [158F],9 the FCGR3A genotype may influence the susceptibility to infections in patients treated with immunosuppressive drugs, which reduce adaptive immunity but not innate immunity. In the present study, IVIg therapy, which is required to maintain the total IgG levels in sera within the normal limit, was appropriately performed after transplantation. Susceptibility to infectious complications may, thus, be determined by a complex interplay between the aforementioned factors. Here, we have proposed that an association between FcγR IIa polymorphism and the effect of rituximab on B cell depletion leads to a significant difference in IgM levels in sera. Since a previous report has demonstrated a critical role of natural IgM in the immediate defense against several antigens,25 it is likely that IgM levels partially influence the incidence of infection after transplantation. In the present study, we have observed a trend such that patients with BSI have lower IgM levels in comparison to those without BSI, although this difference in IgM levels was not found to be statistically significant. Thus, the reduction in the IgM levels in sera observed upon B cell depletion by rituximab may be attributed to the susceptibility to severe infections in association with FCGR2A polymorphism.
As we reported previously, the affinity of FcγRIIIa for IgG3, an important aspect of innate immune defense against microbes, is critical under immunosuppressive conditions after LDLT.24 Since the affinity of FcγRIIIa for IgG3 is much higher than that of FcγRIIa,9 FCGR3A SNP is likely to have a stronger association with innate immune defense. The combination of polymorphisms in FCGR2A influencing the effect of rituximab and FCGR3A influencing the innate defense activity might be a predisposing factor for infectious complications and could predict mortality after ABOi LT. This study provides a foundation for further prospective studies on a larger scale.
In conclusion, we demonstrated that the effects of rituximab on B cells were more profound in FCGR2A [131H/H] than in FCGR2A [131H/R or R/R] recipients of ABOi LDLT. The higher incidence of infectious complications in FCGR2A [131H/H] recipients and their poor prognosis could potentially be attributed to this polymorphism. The combination of FCGR2A and FCGR3A SNPs further stratifies the incidence of infectious complications, ie, FCGR2A [131H/H] and FCGR3A [158F/F or F/V] displayed the highest incidence of infectious complications after ABOi LDLT, suggesting that strict management might be required to improve outcomes. Thus, SNPs of FCGR2A and FCGR3A are likely to be predisposing factors for severe infectious complications after ABOi LDLT, despite the lack of a significant influence of these SNPs on the reduction of antiblood group A/B antibodies. This study can be treated as a good foundation, based on which, further analyses with larger cohorts can be carried out.
Supplementary Material
Footnotes
Published online 25 May, 2017.
The authors declare no conflicts of interest.
This study was partially funded by the Research Program on Hepatitis from Japan Agency for Medical Research and development, AMED (15fk0210016h003).
H.O., H.S., and Y.T. designed the study. H.T., V.S., S.S., and H.S. performed individual genotyping. T.O., T.K., K.I., K.I., H.T., M.O, H.T., S.S., H.S., and H.O. participated in treatment the patients. H.S., Y.T., H.T., and S.S. analyzed the data. H.O. and H.S. wrote the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 2.Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–3908. [PubMed] [Google Scholar]
- 3.Lo P, Sharma A, Craig JC, et al. Preconditioning therapy in ABO-incompatible living kidney transplantation: a systematic review and meta-analysis. Transplantation. 2016;100:933–942. [DOI] [PubMed] [Google Scholar]
- 4.Egawa H, Teramukai S, Haga H, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 2014;14:102–114. [DOI] [PubMed] [Google Scholar]
- 5.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Edberg JC, Redecha PB, et al. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koene HR, Kleijer M, Algra J, et al. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–1114. [PubMed] [Google Scholar]
- 9.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. [DOI] [PubMed] [Google Scholar]
- 10.Warmerdam PA, van de Winkel JG, Gosselin EJ, et al. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32). J Exp Med. 1990;172:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders LA, Feldman RG, Voorhorst-Ogink MM, et al. Human immunoglobulin G (IgG) Fc receptor IIA (CD32) polymorphism and IgG2-mediated bacterial phagocytosis by neutrophils. Infect Immun. 1995;63:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. [DOI] [PubMed] [Google Scholar]
- 13.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. [DOI] [PubMed] [Google Scholar]
- 14.Ahlgrimm M, Pfreundschuh M, Kreuz M, et al. The impact of Fc-gamma receptor polymorphisms in elderly patients with diffuse large B-cell lymphoma treated with CHOP with or without rituximab. Blood. 2011;118:4657–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dall'Ozzo S, Tartas S, Paintaud G, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004;64:4664–4669. [DOI] [PubMed] [Google Scholar]
- 16.Veeramani S, Wang SY, Dahle C, et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118:3347–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghesquières H, Cartron G, Seymour JF, et al. Clinical outcome of patients with follicular lymphoma receiving chemoimmunotherapy in the PRIMA study is not affected by FCGR3A and FCGR2A polymorphisms. Blood. 2012;120:2650–2657. [DOI] [PubMed] [Google Scholar]
- 18.Farag SS, Flinn IW, Modali R, et al. Fc gamma RIIIa and Fc gamma RIIa polymorphisms do not predict response to rituximab in B-cell chronic lymphocytic leukemia. Blood. 2004;103:1472–1474. [DOI] [PubMed] [Google Scholar]
- 19.Dornan D, Spleiss O, Yeh RF, et al. Effect of FCGR2A and FCGR3A variants on CLL outcome. Blood. 2010;116:4212–4222. [DOI] [PubMed] [Google Scholar]
- 20.van Meerten T, van Rijn RS, Hol S, et al. Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Cancer Res. 2006;12:4027–4035. [DOI] [PubMed] [Google Scholar]
- 21.Alcoceba M, Sebastian E, Marin L, et al. HLA specificities are related to development and prognosis of diffuse large B-cell lymphoma. Blood. 2013;122:1448–1454. [DOI] [PubMed] [Google Scholar]
- 22.Byrd JC, Kitada S, Flinn IW, et al. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99:1038–1043. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto H, Ide K, Tanaka Y, et al. Different sensitivity of rituximab-treatment to B-cells between ABO-incompatible kidney and liver transplantation. Hum Immunol. 2016;77:456–463. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu S, Tanaka Y, Tazawa H, et al. Fc-Gamma receptor polymorphisms predispose patients to infectious complications after liver transplantation. Am J Transplant. 2016;16:625–633. [DOI] [PubMed] [Google Scholar]
- 25.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. [DOI] [PubMed] [Google Scholar]
- 26.Cavazzuti I, Serafini G, Busani S, et al. Early therapy with IgM-enriched polyclonal immunoglobulin in patients with septic shock. Intensive Care Med. 2014;40:1888–1896. [DOI] [PubMed] [Google Scholar]
- 27.Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. [DOI] [PubMed] [Google Scholar]
- 28.Ishiyama K, Ohdan H, Ohira M, et al. Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology. 2006;43:362–372. [DOI] [PubMed] [Google Scholar]
- 29.Marino J, Paster JT, Trowell A, et al. B cell depletion with an anti-CD20 antibody enhances alloreactive memory T cell responses after transplantation. Am J Transplant. 2016;16:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


