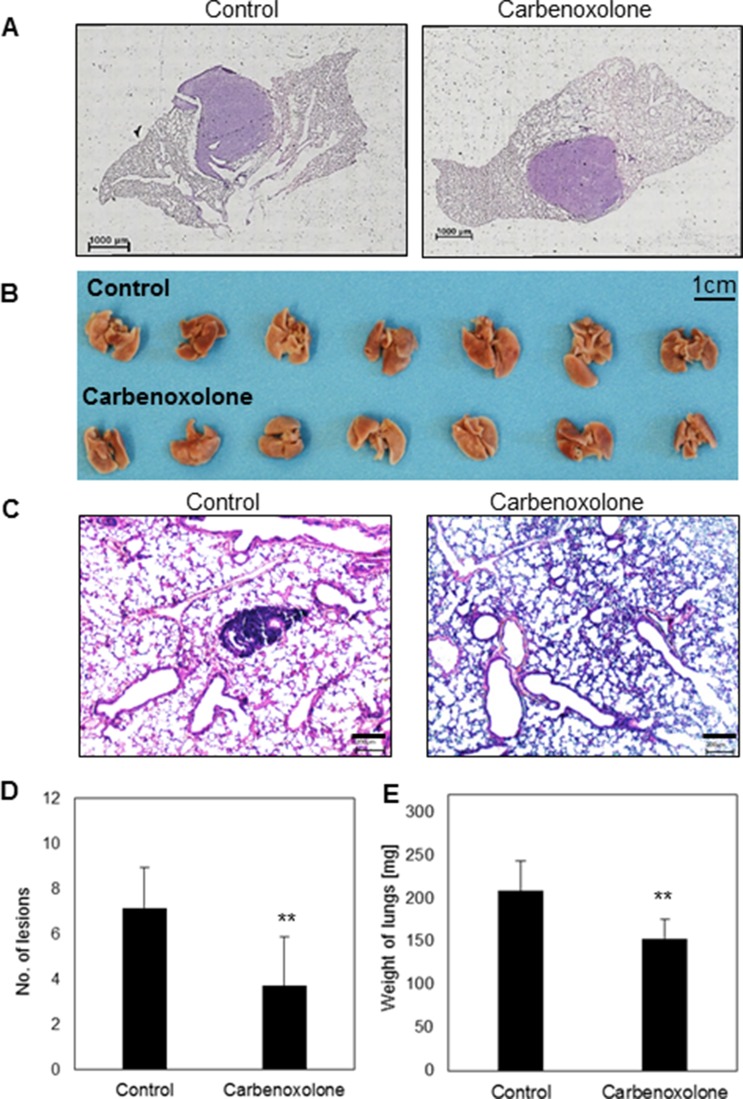
Figure 4. Effect of Carbenoxolone in orthotopic primary growth and tail vein injection.
(A) An orthotopic model- representative histologic sections of LLC tumor in the left lung of C57BL/6 mice untreated (left) and treated (right) with 10 mg/Kg/d of Carbenoxolone for 17 days. Drug was administered 3 days after cancer cells were injected into the lung. (B) Tail vein systemic injections on C57BL/6 mice. Mice were pre-treated i.p. with 50 mg/kg Carbenoxolone q.o.d., (PBS as control). On the 5th day all mice were injected IV with LLC cells (5 × 106). On the 8th day dose was reduced to 40 mg/kg. End point was 21 days after treatment started due to the death of a mouse from the control group, and lungs resected (C) Representative histologic sections of lungs of control (left) and Carbenoxolone treated (right) mice after tail vein experiment post H&E staining (4×). (D) Number of lesions found in lungs of untreated mice compared with lesions found in lungs of mice treated with Carbenoxolone, p = 0.006. (E) Weight of Lungs of untreated mice compared with that of mice under Carbenoxolone treatment, p = 0.004. n = 8. **p ≤ 0.01