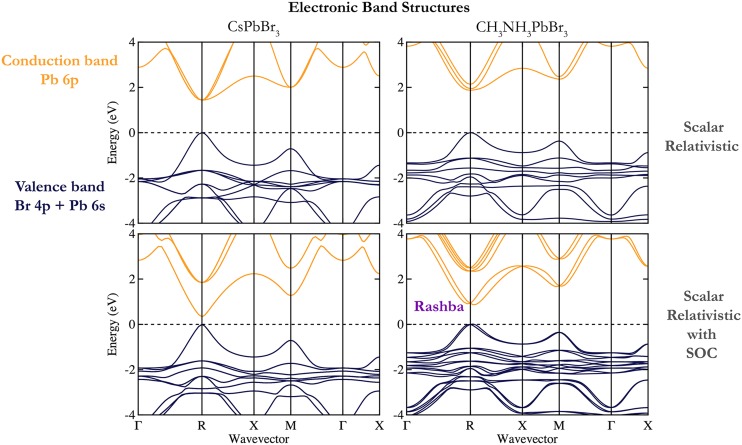
FIG. 3.
The electronic band structures of the inorganic perovskite and hybrid perovskite in the cubic phase. An effect of the organic cation is to widen the bandgap located at the R point due to the larger lattice constant. Spin-orbit coupling reduces the bandgap in both materials. The presence of in the hybrid perovskite results in a non-centrosymmetric crystal, with an associated relativistic Rashba-Dresselhaus splitting of the lower conduction band. While the labels of the special points are those of the cubic perovskite structure (space group ), the static model of the hybrid perovskite formally has P1 symmetry. Points equivalent for a cubic crystal (e.g., ; ; ) are inequivalent here.