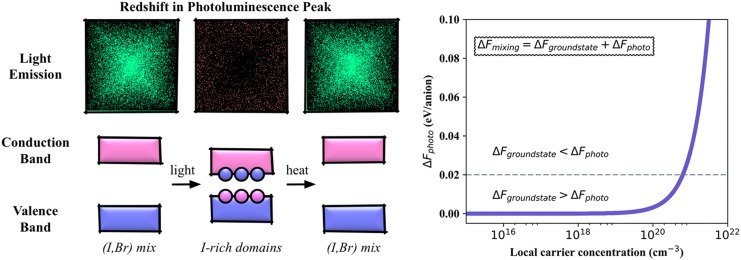
FIG. 4.
Ion transport occurs in halide perovskites: they are mixed ionic-electronic conductors. The vacancy-mediated diffusion of halide anions has been associated with both the current-voltage hysteresis of solar cells and the rapid interchange between iodide, bromide, and chloride materials. The microscopic origin of the reversible ion segregation observed in mixed (Br,I) systems remains unresolved and a subject of debate. Alloyed materials have been found to phase separate upon illumination, but recover their initial state when the light source is removed. The phase separation is associated with a striking red-shift in the photoluminescence spectra. A statistical mechanical analysis of the ground-state DFT calculations suggested a large miscibility gap,86 while the charge carriers generated upon illumination can provide an additional driving force for the phase separation.87 The results from a simple thermodynamic model are shown in the right panel, where the free energy of mixing contains contributions from the ground state () with an additional component due to the difference in bandgaps between the mixed (I,Br) and phase separated I-rich phases (). The latter contribution requires local carrier concentrations approaching 1021 cm−3 to make a substantial contribution to the overall mixing energy.