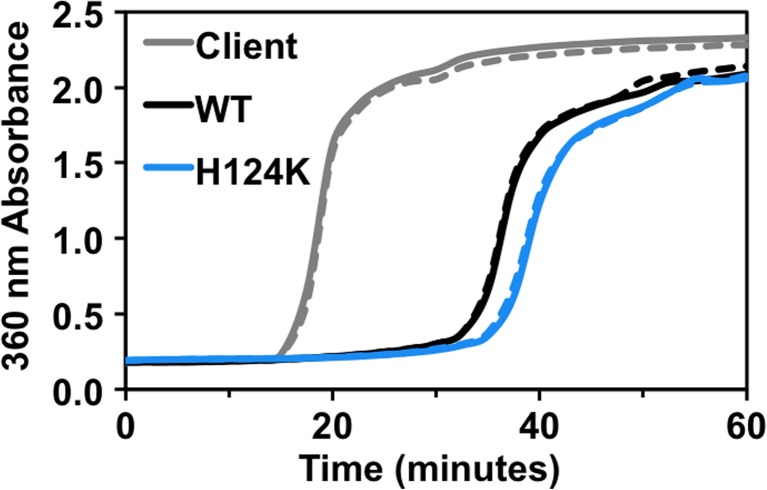
Fig. 3.
Low pH-mimicking mutant of HSPB1 is not highly activated relative to WT-HSPB1. WT and H124K HSPB1 delay aggregation of a model client protein, α-lactalbumin. The final sHSP and client concentrations were 60 and 600 μM, respectively, and aggregation of α-lactalbumin was induced by addition of 20 mM DTT. Duplicate samples are shown. Absorbance at 360 nm was measured over time at 37 °C. The H124K mutant delays aggregation of α-lactalbumin slightly longer than WT protein. This contrasts with the dramatic increase in chaperone activity reported for the analogous H104 mutation in HSPB5 (Rajagopal et al. 2015b)