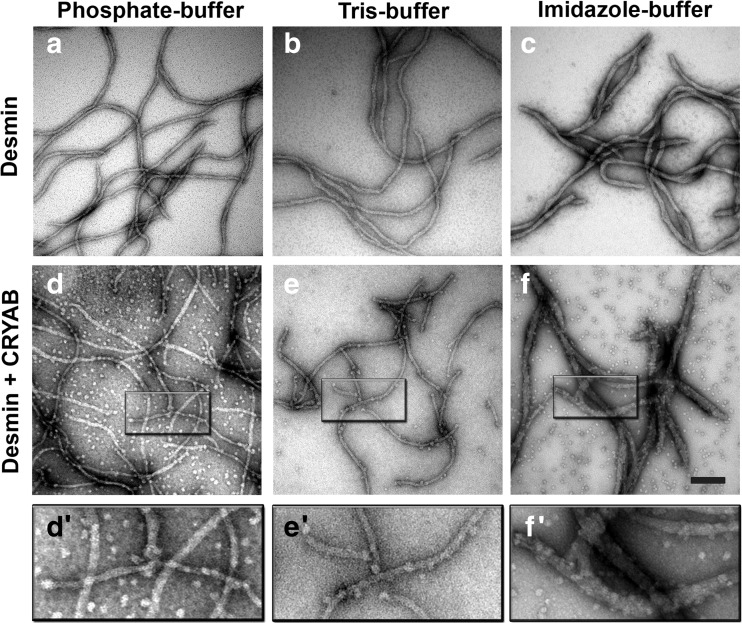
Fig. 1.
Buffer-dependent desmin filament morphology influences association of CRYAB. The filament structure of negatively stained desmin samples in the presence and absence of CRYAB was compared in “Phosphate-buffer” (a, d), “Tris-buffer” (b, e) and “Imidazole-buffer” (c, f) systems. Assembly reactions were stopped at 60 min by addition of 0.1% (v/v) glutaraldehyde prior to electron microscopy. The desmin intermediate filament width was preserved in “Phosphate-buffer” (11.5 nm) and “Tris-buffer” (12.3 nm), but was significantly wider in “Imidazole-buffer” (25.8 nm). In the Tris buffer, CRYAB bound along short desmin filaments in a regular manner (e), whilst less binding was observed for phosphate buffer and imidazole buffer (d’, f’). Insets show the position of CRYAB oligomers aligned along desmin filaments (see arrows, d’–f’). Scale bar = 100 nm