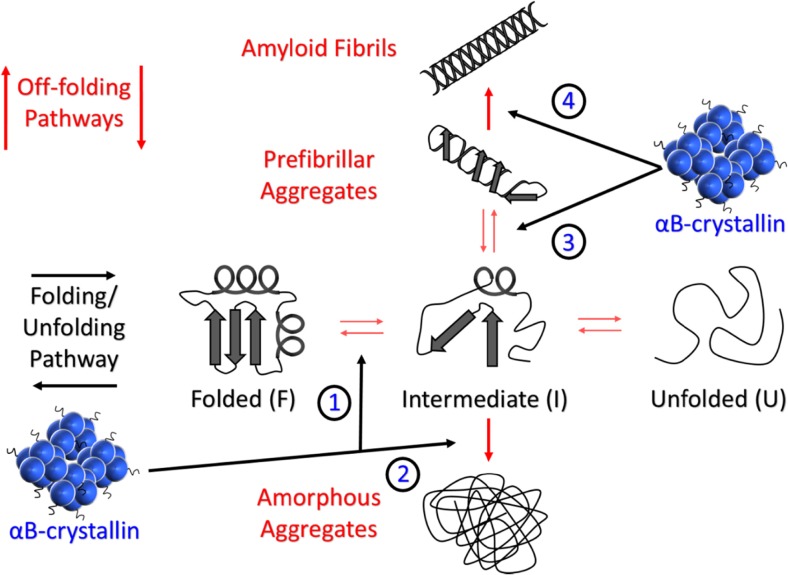
Fig. 1.
Schematic of the protein folding/unfolding pathway (horizontal) and the off-folding pathways (vertical). The folding/unfolding pathway depicts both folded (F) and unfolded (U) states whilst the intermediate (I) represents the partially folded state(s) between these two extremes which is characterised by having elements of secondary structure and has the potential to enter the off-folding pathways to form amorphous aggregates and/or amyloid fibrils. The oligomeric sHsp αBc is depicted with small black ‘squiggly’ protrusions representing the solvent-exposed and flexible C-terminal extension. The various junctures at which αBc interacts on the folding/unfolding and off-folding pathways (Cox et al. 2014; Treweek et al. 2015) are indicated with black arrows labelled 1, 2, 3 and 4. Briefly, 1. Interaction with destabilised native-like species, 2. Interaction with intermediate species to prevent or delay amorphous aggregation, 3. Interaction with intermediate species to prevent or delay the generation of prefibrillar aggregates and also to dissociate prefibrillar/oligomeric species back to an intermediate or native state, 4. Binding to amyloid fibrils in order to stabilise them and prevent further elongation and fragmentation which may lead to secondary nucleation