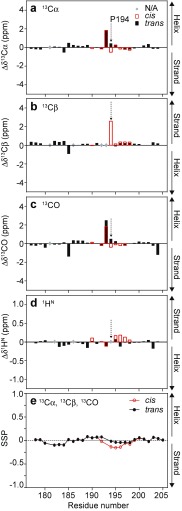
Fig. 3.
Secondary chemical shifts, Δδ, probe residual structure in the C-terminal region of HSP27. Secondary chemical shifts are defined as the difference between the experimentally measured chemical shift of a given nuclei in a given residue and the same nuclei from the same residue in a random coil conformation, typically extracted from a database of known values. Shown here are the secondary chemical shifts for 13Cα (a), 13Cβ (b), 13CO (c), and 1HN (d) nuclei in HSP27. The arrow depicts the position of P194 in the amino acid sequence. Note that artificially large values are observed for G193 due to its i-1 position to P194. e The 13C chemical shifts from the CTR of HSP27 were used as input to calculate the secondary structure propensity (SSP) of this region. The y-axis depicts the propensity to populate α-helical (+1.0) or β-strand (−1.0) conformations, with a value of, e.g., −0.2 corresponding to 20% β-strand. The cis-P194 state is shown in red and the trans-P194 state in black