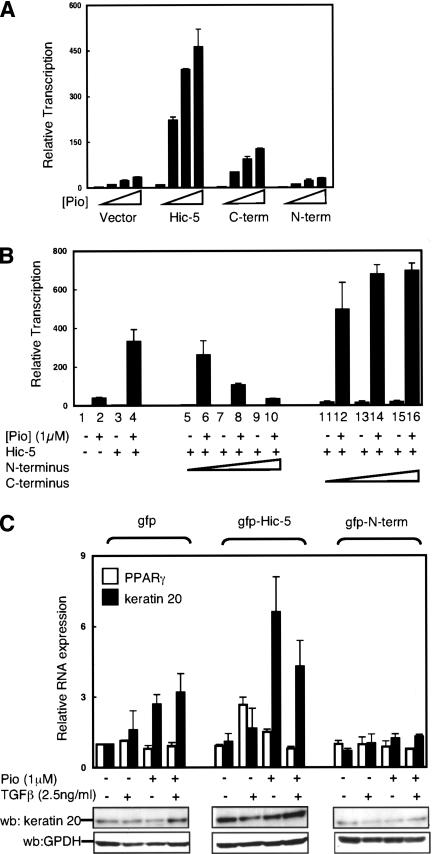
Figure 4.
TGFβ enhances PPARγ induction of keratin 20, while dominant-negative Hic-5 blocks it. (A) Cos-1 cells were transiently cotransfected with 50 ng expression vector for PPARγ, 50 ng luciferase reporter plasmid with three PPARγ response elements (DR1-luc) in the presence of 250 ng gfp-Hic-5, 400 ng gfp-C terminus Hic-5(209-444), gfp-N terminus Hic-5(1-208) expression vectors, or gfp expressing vector as a control. Cells were treated with increasing concentrations of pioglitazone (Pio) as shown. Cell lysate were then analyzed for relative transcriptional activity, as described in Figure 1A. (B) Cos-1 cells were transiently tranfected and treated as described in A. To determine potential dominant-negative activities, Hic-5 expressing vector (100 ng) was cotransfected with increasing amount of either gfp-N terminus Hic-5 or gfp-C terminus Hic-5(100-400 ng). (C) Moser cells transduced with retroviral expressing Hic-5, dominant-negative Hic-5 (gfp-N terminus), or gfp were incubated with or without TGFβ or PPARγ ligand (pioglitazone). After 2 d, total RNA and protein were extracted. (Top) Relative keratin 20 and PPARγ mRNA levels were analyzed by real-time RT-PCR. Values of keratin 20 and PPARγ were normalized to expression level without treatment in control cells, corrected to β-actin and were mean ± SE of three different experiments. (Bottom) Protein lysates were extracted and separated by SDS-PAGE gels. Keratin 20 and GPDH levels were analyzed by Western blot using keratin 20 and GPDH antibodies respectively. GPDH levels are shown as a loading control.