Figure 2.
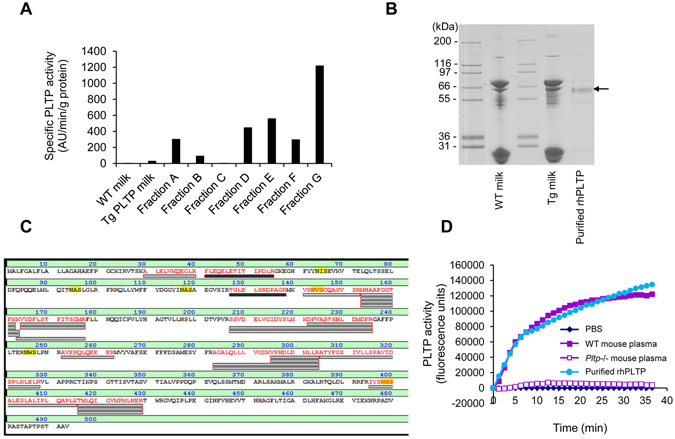
Purification and characterization of recombinant human PLTP from PLTPTg rabbit milk. (A) After FPLC elution of clarified milk proteins on a Heparin Sepharose 6 Fast Flow column, using a discontinuous NaCl gradient with 100 mM step increases, different fractions (A–G) were collected at different NaCl concentrations and analyzed for specific PLTP activity. The fraction exhibiting the highest specific PLTP activity (G) was eluted at the 300 mM NaCl step. (B and C) Electrophoretic analysis of purified rhPLTP (B) shows a sharp, 60 kDa band which was analyzed by MALDI-TOF/TOF MS and a database search. The protein (arrow) was identified as human PLTP according to the matched peptides which are shown in bold red (C). (MW: 54.7 kDa. Number of matching peptides: 24. Sequence coverage: 39.8%. Score: 182 for a significant matching score above 50). (D) Comparison of the phospholipid transfer activities of active purified rhPLTP, wild-type (WT) mouse plasma, Pltp−/− mouse plasma, and PBS buffer. The active rhPLTP shows phospholipid transfer activity of the same magnitude as that in WT mouse plasma.
