Figure 6.
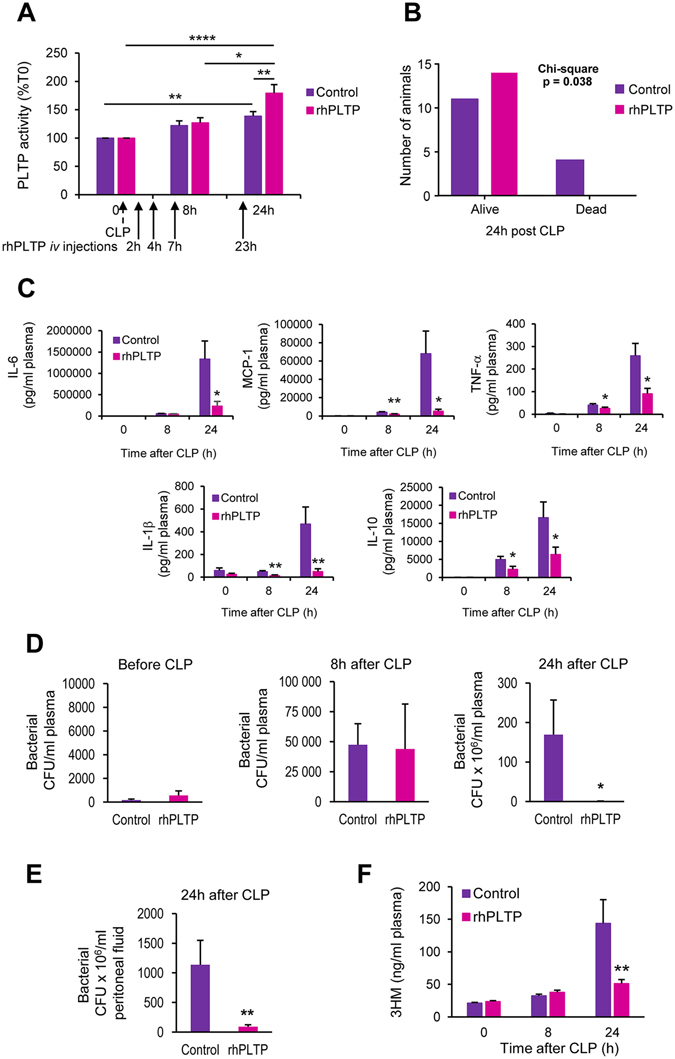
rhPLTP retained its protective effects in WT mice with polymicrobial sepsis. WT mice received iv injections of vehicle (control) or rhPLTP (25 µg in a volume of 200 µl) 2 h, 4 h, 7 h and 23 h after CLP. (A) Repeated iv injections of rhPLTP after CLP induced a significant increase in plasma PLTP activity 24 h after CLP. PLTP activity was measured in plasma before, 8 h and 24 h after CLP and expressed as a percentage of T0 mouse plasma activity (n = 12–14 mice per group, Kruskal-Wallis test with Dunn’s multiple comparisons test). (B) Repeated iv injections of rhPLTP increased mouse survival after CLP. Numbers of alive and dead animals were compared using the χ2 test (n = 14–15 mice per group). (C) Repeated iv injections of rhPLTP prevented the production of cytokines (IL-6, MCP-1, TNF-α, IL-1β, IL-10) after CLP. Plasma samples, harvested from WT mice before CLP, 8 h and 24 h after CLP, were assayed using a Milliplex mouse cytokine panel (n = 12–14 mice per group, Mann-Whitney test). (D and E) Intravenous rhPLTP administration reduced bacterial burdens in blood and peritoneal lavages of WT mice with polymicrobial sepsis. Colony-forming units (CFU) were determined in blood samples (D) before CLP, 8 h and 24 h following CLP and in peritoneal lavage fluids (E) 24 h after CLP (n = 12–14 mice per group, Mann-Whitney test). (F) Intravenous rhPLTP administration significantly reduced LPS levels in WT plasma after CLP. LPS concentrations were determined by direct quantitation of 3-hydroxymyristate (3HM) over a 24 h period following CLP in plasma from WT mice (n = 12–14 mice per group, Mann-Whitney test). Data are means ± sem. *P < 0.05, **P < 0.01.
