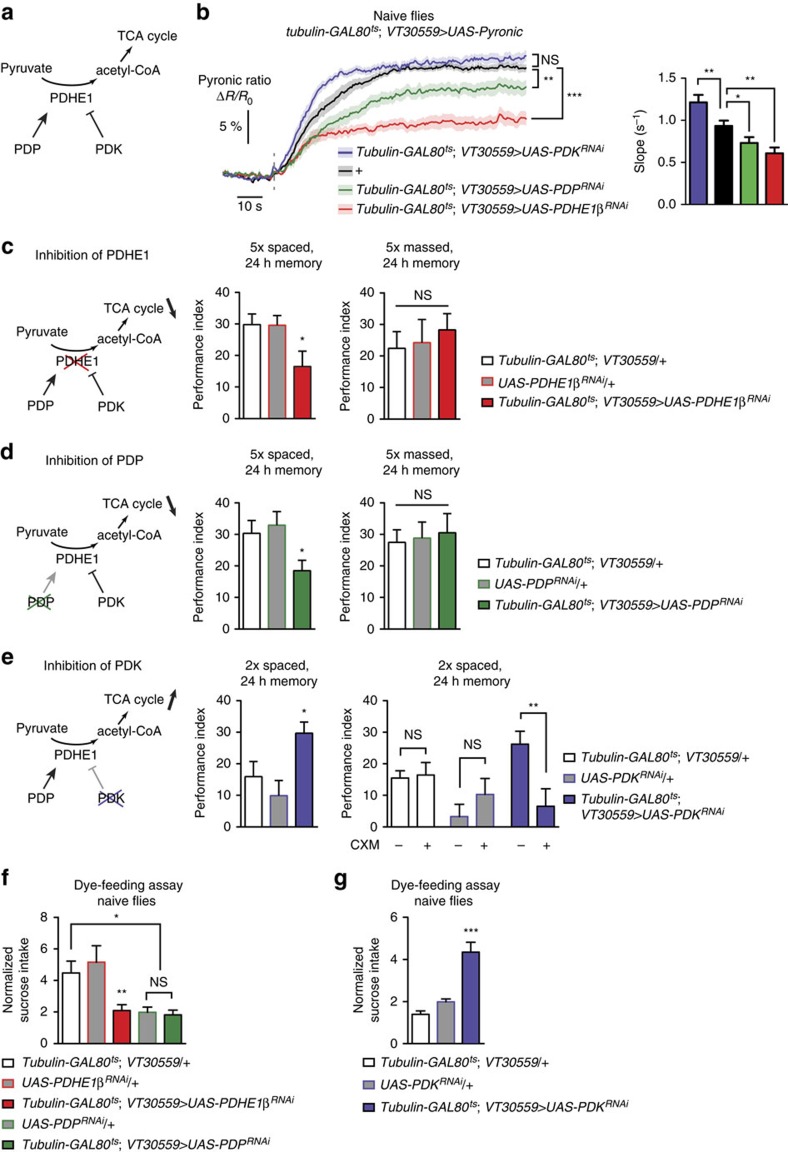
Figure 4. Enhanced energy flux in MB neurons following spaced training is necessary and sufficient for LTM formation.
(a) Representation of the action of PDHE1, PDP and PDK. In mitochondria, PDHE1 catalyzes the conversion of pyruvate to acetyl-coA, which enters the tricarboxylic acid cycle (TCA). PDHE1 can be inactivated through phosphorylation by PDK. In contrast, PDP can activate a phosphorylated PDHE1. (b) Imaging of pyruvate accumulation following azide application in naive flies co-expressing Pyronic and RNAi against either the β subunit of PDHE1 (PDHE1β), PDP or PDK in MB neurons at adult stage. The plateau value corresponding to sensor saturation was significantly different between the different conditions (n=11–17; F3,100=26.8, P<0.0001). The slope of accumulation was also significantly different: pyruvate accumulation was faster with PDK inhibition, and slower with PDP and PDHE1 inhibition, compared to control flies (F3,100=12.24, P<0.0001). (c) Inhibition of PDHE1β in adult MB neurons impaired 24-h memory after 5 × spaced training (n=15–19; F2,49=3.89, P=0.028), but not after 5 × massed training (n=10–11; F2,31=0.26, P=0.77). (d) Inhibition of PDP in adult MB neurons impaired 24-h memory after 5 × spaced training (n=14–15; F2,43=3.95, P=0.027), but not after 5 × massed training (n=10–11; F2,31=0.088, P=0.92). (e) Flies expressing an RNAi against PDK in MB neurons exclusively at the adult stage showed increased memory after two spaced training cycles as compared to their genotypic controls (n=10–12; F2,31=5.16, P=0.012). The memory of these flies, but not of their genotypic controls, was sensitive to CXM treatment, which is a hallmark of LTM (tubulin-GAL80ts; VT30559/+: n=17 and 14; t29=0.22; P=0.83; +/UAS-PDKRNAi: n=12 and 13: t23=1.09; P=0.29; tubulin-GAL80ts; VT30559/UAS-PDKRNAi: n=18 and 19; t35=2.87; P=0.007). (f,g) Inhibition of PDHE1β in adult MB neurons of naive flies resulted in a marked decrease of sucrose intake compared to the two genotypic controls (f). For PDP inhibition, one of the genotypic controls showed also low sucrose intake, which hampers the interpretation of the data. (n=18 groups of 5 flies; F4,89=6.24, P=0.0002). Inhibition of PDK induced a strong increase in sucrose intake compared to the genotypic controls (g; n=12 groups of five flies; F2,34=28.7, P<0.0001).