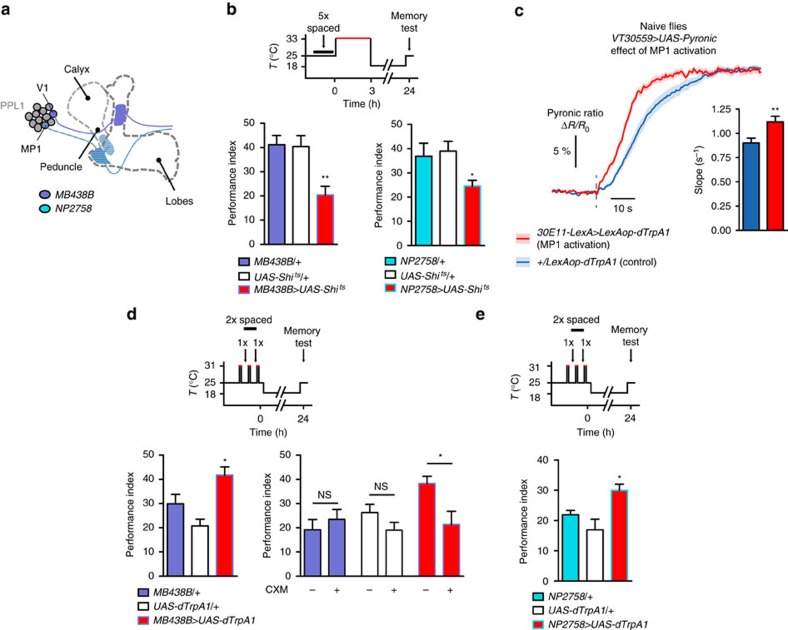
Figure 5. Dopamine input on MB neurons triggers increased energy flux and LTM formation.
(a) Illustration of the dopaminergic neurons in the PPL1 cluster that are targeted by the MB438B and NP2758 drivers. Both drivers target MP1 neurons. Expression patterns of the driver lines used in this study are available online on several public databases. See Experimental Procedures for details. (b) Flies were submitted to a 3 h period at 33 °C following spaced training to block the output of Shits-expressing neurons. Flies expressing Shits through either the MB438B or NP2758 driver showed an LTM defect as compared to their genotypic controls (MB438B: n=7–11 F2,26=9.23, P=0.0011; NP2758: n=8–11, F2,26=4.40, P=0.023). (c) Flies were subjected to a thermal treatment that consisted of two 3-min periods at 33 °C separated by 5 min. Pyruvate accumulation following azide application was measured 2–3 min later. 30E11-LexA>LexAop-dTrpA1 flies (n=10) were compared to +/LexAop-dTrpA1 genotypic control flies (n=13). The slope of pyruvate accumulation was higher in flies with activated MP1 neurons (t44=2.87; P=0.0063). (d) Flies were subjected to two cycles of spaced training, interspersed by 1-min periods at 31 °C to activate dTrpA1-expressing neurons. Flies expressing dTrpA1 through the MB438B driver showed enhanced 24 h-memory compared to their genotypic controls (MB438B: n=8–10, F2,26=10.19, P=0.0006). The memory of these flies, but not of their genotypic controls, was sensitive to cycloheximide (CXM) treatment, which is a hallmark of LTM (MB438B/+: n=12; t22=0.73; P=0.47; +/UAS-dTrpA1: n=12; t22=1.56; P=0.13; MB438B/UAS-dTrpA1: n=12; t22=2.72; P=0.012). (e) Same training protocol as in d. Flies expressing dTrpA1 through the NP2758 driver showed enhanced 24 h-memory compared to their genotypic controls (NP2758: n=12, F2,35=6.89, P=0.0032).