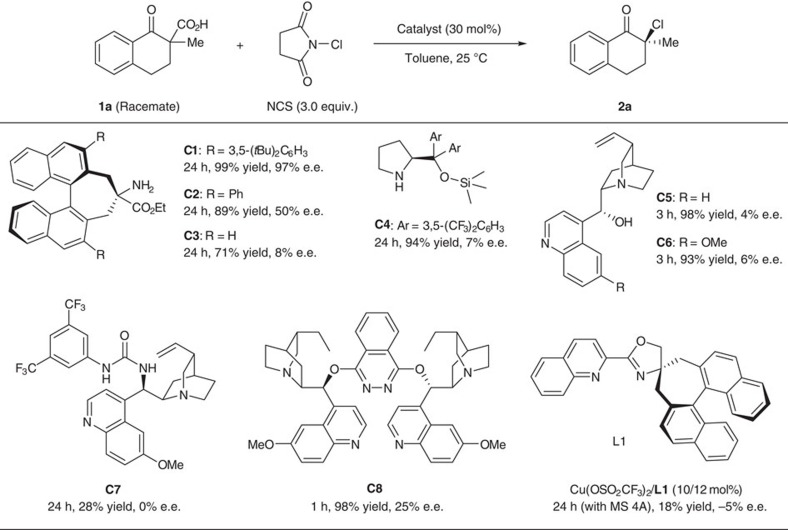
Figure 2. Screening of chiral catalysts for enantioselective decarboxylative chlorination.
β-Ketocarboxylic acids were treated with NCS (3.0 equiv.) in the presence of chiral catalyst (30 mol%) and the mixture was stirred for 24 h in the dark, unless otherwise noted. The reactions with C5 and C6 were carried out for 3 h while the reaction with C8 was carried out for 1 h. The reaction using the complex of Cu(OSO2CF3)2 (10 mol%) and L1 (12 mol%) (Cu(OSO2CF3)2/L1) was performed in the presence of MS 4 A. In all cases, the yield of 2a corresponds to the isolated yield of the purified compound, and the e.e. for each compound was determined by chiral high-performance liquid chromatography (Et=ethyl group, tBu=tert-butyl group).